4636
Alterations in brain white matter structural network in children with seizure and cognitive dysfunction
Yannan Cheng1, Chao Jin2, Xianjun Li2, Congcong Liu2, Miaomiao Wang2, Huifang Zhao2, Xiaoyu Wang2, Yuli Zhang2, Fan Wu2, Mengxuan Li2, Cong Tian2, Peiyao Chen2, Xiaocheng Wei3, Jianxin Guo2, and Jian Yang2
1Department of Radiology, the First Affiliated Hospital, Xi’an Jiaotong University, Xi'an, China, Xi'an, China, 2the First Affiliated Hospital, Xi’an Jiaotong University, Xi'an, China, Xi'an, China, 3MR Research China, GE Healthcare, Bei Jing, People's Republic of China, Xi'an, China
1Department of Radiology, the First Affiliated Hospital, Xi’an Jiaotong University, Xi'an, China, Xi'an, China, 2the First Affiliated Hospital, Xi’an Jiaotong University, Xi'an, China, Xi'an, China, 3MR Research China, GE Healthcare, Bei Jing, People's Republic of China, Xi'an, China
Synopsis
Seizures are the most common disease in children and some types like epilepsy usually cause many serious co-morbidities such as cognitive dysfunction. We detailed the alterations of brain white matter network in children with seizure and cognitive dysfunction by comparing the difference of network properties and connectivity strength between the patient and control groups. Here, we found that children with seizure and cognitive dysfunction show a suboptimal brain organization with reduced information integration ability, and abnormalities in multiple intrinsic functional connectivity may be the structural basis for cognitive impairment in patients with seizures.
Purpose
Seizures are the most common disease in children and some types like epilepsy usually cause many serious co-morbidities1,2. Among these, cognitive dysfunction is the most common and troublesome2. Studies have shown that cognitive functions benefit from the efficient communication between different brain regions in a large-scale network3. And functional and structural studies both have found that distinct seizures with cognitive impairment manifests as abnormal brain network connectivity and network topology changes in adult4-6. While less is known regarding alterations of brain white matter structural network in children with seizure and cognitive disfunction, which was evaluated in this study.Materials and Methods
The Institutional Review Broad of the first author’s affiliation approved this study and written informed consent were obtained from parents of the children. Subjects Forty-eight children with seizure and no abnormality on MRI participated in the study. Cognitive ability was assessed by the Chinese Wechsler Young Children scale of Intelligence (C-WYCSI) for children aged 4 to 6 years and Chinese Wechsler Intelligence Scale for Children (C-WISC) aged 6 to 18 years. Then, all subjects were divided into control (seizure with normal cognitive function) (n=34) and patients (seizure with cognitive dysfunction) (n=14) according to full scale intelligence quotient (FSIQ). Cognitive dysfunction was defined as FSIQ <85 scores. MR Protocols All subjects were examined by using a 3.0T scanner (Signa HDxt, General Electric Medical System, Milwaukee, WI, USA) with an 8-channel head coil. Data acquisition included three-dimensional fast spoiled gradient-echo T1-weighted sequence (TR/TE, 10.2ms/4.6ms; NEX, 1; isotropic 1×1×1mm3; FOV, 24cm) and transverse fast spin-echo T2-weighted sequence (TR/TE, 4200ms/113ms; NEX of 1.5; matrix, 320×320; thickness, 4mm; FOV, 24cm), followed by a DTI (30 directions; b value, 600s/mm2; TR/TE, 11000ms/67.4ms; NEX, 1; thickness, 2.5mm; FOV, 24cm; matrix, 172×172). Data and statistical analysis DTI and T1 data were processed through standard pipeline in PANDA software. The brain connectivity matrix was constructed based on fiber number, using AAL atlas as a reference. A network-based statistic (NBS) was used to identify the difference in connectivity strength between the two groups. Network properties were computed by Gretna software using graph theory, including (1) global network properties: small-worldness (σ), global efficiency and shortest path length; (2) nodal network properties: efficiency and shortest path length. SPSS 18.0 (SPSS, Chicago, IL, USA) was used to find the difference in network properties between two groups. p<0.05 was considered as statistically significant difference.Results
Forty-eight children were included in this study. Significant differences presented in FSIQ scores between the two groups, while no differences were observed in age at MRI scan, sex, age of onset and duration of seizures (Table 1).All children demonstrated small-world property of the white matter networks (σ>1). Both global and nodal results showed that increased shortest path length and reduced efficiency in patients group compared with control group. Furthermore, we also found these regions mainly consisted of (1) bilateral default mode network: superior frontal gyrus (medial), anterior cingulate gyrus, middle cingulate gyrus, posterior cingulate gyrus and precuneus; (2) left frontoparietal network (superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus triangular and rolandic operculum); (3) visual network: bilateral fusiform, left middle temporal gyrus and lingual gyrus, right inferior occipital gyrus and heschl’s gyrus (Figure 1).
From the results of NBS analysis, forty-three connections demonstrated reduced connectivity (P<0.001, corrected) in patients group relative to control group. Consistent with the previous findings, these connections also mainly distributed in bilateral default mode network, left frontoparietal network, and right visual network (Figure 2). None of the connections showed increased connectivity in patient group.
Discussion
All children's structural brain white matter network showed a small world topology, which was consistent with previous studies7. Then, we also found that increased shortest path length and reduced global efficiency in patients group relative to control group. Previous studies have found that the above parameters usually represent the ability to integrate information in the whole brain, indicating that altered organization of brain white matter networks may impact the ability to effectively integrate information from different brain regions in children with seizure and cognitive dysfunction7. In addition, children with seizure and cognitive dysfunction showed reduced nodal efficiency and increased path length affecting many regions. And according to Yeo’ study8, we found that the affecting area primarily located in the bilateral default mode network, followed by the left frontoparietal network and the right visual network. Last, we discovered that forty-three connections demonstrated reduced connectivity in patient group. Consistent with the above results, these connections also mainly distributed in above functional network. Based on these findings, we can induce that children with seizure and cognitive dysfunction may present as impairment in language, visual function and some psychiatric disorders 8.Conclusion
Children with seizure and cognitive dysfunction show a suboptimal brain organization with reduced information integration ability, and abnormalities in multiple intrinsic functional connectivity may be the structural basis for cognitive impairment in patients with seizures.Acknowledgements
This study was supported by the National Natural Science Foundation of China (81901516, 81971581, 81901823, 81771810 and 51706178), Shaanxi Provincial Innovation Team (2019TD-018), National Key Research and Development Program of China (2016YFC0100300), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438), the Project Funded by China Postdoctoral Science Foundation (No. 2019M653659), and the Natural Science Basic Research Plan in Shaanxi Province of China (No.2019JQ-198).References
- Sharma A. Seizures and Epilepsy in Children. The Indian Journal of Pediatrics, 2013, 80(11):925-935.
- Holmes G L. Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disorders, 2015, 17(2): 101-116.
- Deary I J, Penke L, Johnson W. The neuroscience of human intelligence differences. Nature reviews neuroscience, 2010, 11(3): 201.
- Vaessen M J, Jansen J F A, Vlooswijk M C G, et al. White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cerebral cortex, 2011, 22(9): 2139-2147.
- Yang H, Zhang C, Liu C, et al. Brain network alteration in patients with temporal lobe epilepsy with cognitive impairment. Epilepsy & Behavior, 2018, 81: 41-48.
- Bonilha L, Tabesh A, Dabbs K, et al. Neurodevelopmental alterations of large‐scale structural networks in children with new‐onset epilepsy[J]. Human brain mapping, 2014, 35(8): 3661-3672.
- Widjaja E, Zamyadi M, Raybaud C, et al. Disrupted global and regional structural networks and subnetworks in children with localization-related epilepsy. American Journal of Neuroradiology, 2015, 36(7): 1362-1368.
- Thomas Yeo B T, Krienen F M, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology, 2011, 106(3): 1125-1165.
Figures
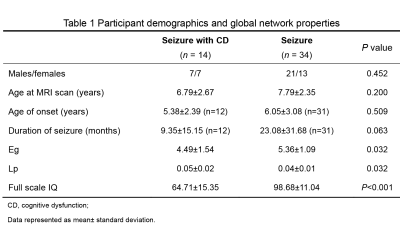
Table 1 Participant demographics and global
network properties
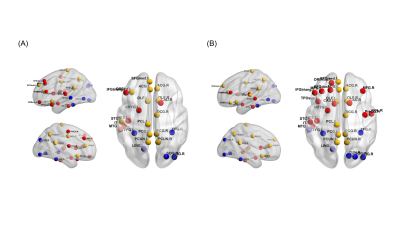
Figure
1 (A) Reduced nodal efficiency and (B) increased path length in children with seizures
and cognitive dysfunction. Yellow, red and blue color respectively represented
default mode network, frontoparietal network and the visual network.
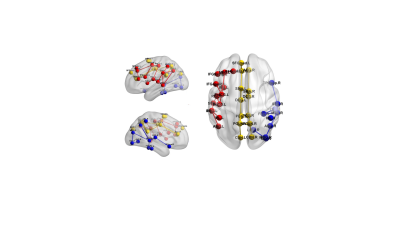
Figure
2 Reduced connectivity strength in children with seizures and cognitive dysfunction.
Yellow, red and blue color respectively represented default mode network, frontoparietal
network and the visual network.