4634
Functional Brain Network Connectivity Patterns in Never and Currently Stunted Young Children in India1Warren Alpert Medical School, Brown University, Providence, RI, United States, 2Advanced Baby Imaging Lab, Hasbro Children's Hospital, Providence, RI, United States, 3Community Empowerment Lab, Lucknow, India, 4Maternal, Newborn, and Child Health Discovery & Tools, Bill & Melinda Gates Foundation, Seattle, WA, United States
Synopsis
Growth stunting is an indicator of poor child development, negatively impacting cognitive performance and overall health until adulthood. While previous studies have investigated links between neurocognitive functioning and resting state network functional connectivity (fc), most have been conducted in developed countries. In our study, we investigate brain network fc at rest in young children (<2 years) living in an Indian region with one of the worst human development indicators. When currently stunted, fewer fc networks were present than in never stunted children, possibly indicating an effect of stunting on brain fc already at a very young age.
Introduction
An estimated 155 million children worldwide are currently stunted, i.e. demonstrating a height-for-age median more than two standard deviations below WHO Child Growth Standards. Most of these children live in low and middle income countries (LMICs), where 14% of childhood deaths are attributable to stunting1. Growth stunting is considered an indicator of child health inequalities, including malnutrition, infectious diseases and cognitive delays - all factors influencing brain development and cognitive ability in later life2. Indeed, several studies have linked growth stunting with lower cognitive performance3, and neurocognitive functioning in adolescents has been successfully predicted by resting state network functional connectivity (rsFMRI)4. Magnetic resonance imaging (MRI) is a universal and objective measure visualizing brain neuroanatomy and functional connectivity of its anatomical regions. MRI has been widely used to investigate children’s neurodevelopment5 but due to a limited number of neuroimaging centers and difficulty in access, most studies have been conducted in developed countries and very few have been taken place in LMICs6. In our study, we are investigating functional connectivity (fc) of brain networks at rest in a cohort of children living in the state of Uttar Pradesh (UP) in India, one of the regions in India with the worst human development indicators7. This study focuses on early childhood (< 2 years old), a period during which the brain undergoes fast and important changes but where typical cognitive assessments are difficult, as higher cognition and language are still actively developing, leaving the true prevalence of developmental difficulties in children aged 0-3 years unknown8. However, interventions during that period appear to be the most efficient to reduce the effects of adversity9. The aim of this work is to provide new insights into differences in brain connectivity in stunted and never stunted young children in India.Methods
MRI data were acquired on 30 currently stunted (15 girls, 14 months mean age, 7-20 months range) and age- and gender-matched 55 never stunted children (26 girls, 12 months mean age, 7-20 months range) during natural sleep on a 3 T Philips Achieva scanner. Height for age z (HAZ) scores were assessed for all children and used for defining never and currently stunted (z score≤-2).Resting state fMRI data acquisition lasted about 6.7 min with the following protocol: TE= 30 ms; TR=2.5 s; voxel resolution= 2.5 x 2.5 x 3 mm3; 40 slices. Structural scans were obtained with standard MPRAGE. Children with any major neurological disorders were excluded from the study. Functional MRI data were first preprocessed (realignment, centering, motion correction, noise removal, scrubbing) with the CONN fMRI toolbox10 on MATLAB and registered to our child study template using FSL FLIRT11 and ANTS12. The CONN fMRI toolbox was used to extract fc values and perform ROI-to-ROI network connectivity analyses using a 32 infant network atlas10 to estimate the impact of stunting for the currently stunted and never stunted groups individually as well as their fc correlation patterns with stunting values within each group. Nuisance covariates and realignment parameter noises were reduced using CompCor13, and fMRI data were band-pass filtered (0.008 < f < 0.09 HZ). As a previous study in the same region of India revealed a significantly higher risk of malnutrition among female children14 and dimorphic connectivity trajectories have been indicated for boys and girls already during infancy15, we controlled all analyses for biological sex and age, and FDR-corrected all results for multiple comparisons at p≤.05.Results
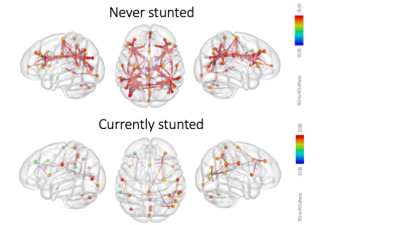
When comparing currently stunted with never stunted children, fewer fc networks were present in the currently stunted group (Figure 1). In addition, while network fc was mainly positive and widely distributed across networks, more negative within network connections were present in the never stunted group.Discussion
While similar fc networks were present at rest in both currently stunted and never stunted children (Figure 1), severely less network fc activity was observed in currently stunted children. In line with these findings, functional network connectivity increased with increasing height for age in both groups, indicating a more fundamental stunting-brain connectivity relationship. However, while our data implies that these relationships are already established at a very young age, it is not yet known whether these differences will have a long lasting impact. The human brain follows distinct developmental trajectories, which occur in tandem with cognitive development16. In turn, developmental delays have further been associated with a range of emotional, behavioral and health problems in later life, with stunted children remaining behind their non-stunted peers in later life17, in turn placing a significant burden on family and society18. As growth stunting is often irreversible after the first two years of life19, early detection and interventions are necessary and needed. We hope that our findings underline the urgency to intervene as early as possible.Acknowledgements
We would like to thank the National Institutes of Health (UH3 ODD023313 and R01 MH087510) and the Bill and Melinda Gates Foundation (OPP11002016) for their continuous support of this work.References
1. World Health Organization (2015). Child growth standards: length/height-for-age. Geneva: World Health Organization. 2. Walker, S. P., Wachs, T. D., Grantham-McGregor, S., Black, M. M., Nelson, C. A., Huffman, S. L., et al. (2011). Inequality in early childhood: risk and protective factors for early child development. The Lancet,378:1325–38. 3. Woldehanna, T., Behrman, J. R., Araya, M. W. (2017). The effect of early childhood stunting on children’s cognitive achievements: evidence from young lives in Ethiopia. Ethiop J Health Dev, 31(2), 75-84. 4. Sripada, C., Rutherford, S., Angstadt, M., Luciana, M., Weigard, A., Hyde, L. H., Heitzeg, M. (2019). Prediction of neurocognition in youth from resting state fMRI. Molecular Psychiatry. 5. Gao et al., Neuroscientist, 2017;23(2):169–84. 6. OECD (2018), Magnetic resonance imaging (MRI) units (indicator). doi: 10.1787/1a72e7d1-en; last accessed Sept. 29 2018. 7. Menon et al. (2018). Understanding the geographical burden of stunting in India: A regression‐decomposition analysis of district‐level data from 2015–16. Matern Child Nutr., 14(4):e12620. 8. Ertem, I., Ozturk, I. (2012). Developmental difficulties in early childhood: prevention, early identification, assessment and intervention in low- and middle-income countries: a review. World Health Organization, ISBN 978 92 4 150354 0 (NLM classification WS 105). 9. Berens, A. D., Nelson, C. A. (2015). The science of early adversity: is there a role for large institutions in the care of vulnerable children? Lancet, 386(9991), 388-398. 10. Whitfield-Gabrieli and Nieto-Castanon (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3),125–141. 11. Smith et al. (2014). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(1):S208-219. 12. Avants et al. (2014). The insight ToolKit image registration framework. Front. Neuroinformatics, 28;8:44. 13. Behzadi, Y., Restom, K., Liau, J., Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based MRI. (2007). NeuroImage, 37(1), 90-101. 14. Srivastada, A., Mahmood, S. E., Srivastava, P. M., Shrotriya, V. P., Kumar, B. (2012). Nutritional status of school-age children – A scenario of urban slums in India. Archives of Public Health, 70(8). 15. Gao, W., Alcauter, S., Smith, J. K., Gilmore, J., Lin, W. (2015). Development of human brain cortical network architecture during infancy. Brain Struct Funct, 220(2), 1173-1186. 16. Silbereis, J. C., et al. (2016). The cellular and molecular landscapes of the developing human central nervous system. Neuron, 89(2), doi: 10.1016/j.neuron.2015.12.008. 17. Walker, S. P., Chang, S. M., Powell, C. A., Grantham-McGregor, S. M. (2005). Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-stunted Jamaican children: prospective cohort study. The Lancet, 366(9499), 1804-1807. 18. Newacheck, P. W., Kim, S. E. (2005). A national profile of health care utilization and expenditures for children with special health care needs. Arch Pediatr Adolesc Med, 159(1), 10-17. 19. Martorell, R., Khan, L. K., Schroeder, D. G. (1994). Reversibility of stunting: epidemiological findings in children from developing countries. European Journal of Clinical Nutrition, 48(1), S45-57.
Figures