4632
Delineation of language network maturation during infancy with multi-modal perfusion and functional MRI1Department of Radiology, The Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Department of Radiology, Beijing Children’s Hospital, Capital Medical University, Beijing, China, 3Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 4Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Human language capabilities are acquired during infancy. We hypothesized that language brain circuit emergence is supported by rapidly increasing regional cerebral blood flow (rCBF) to meet the metabolic demands. However, the spatiotemporal distribution of rCBF in language network during infancy is unknown. In this study, we acquired resting-state fMRI and pCASL perfusion MRI from 48 infants aged 0-24months for identifying language network and quantifying rCBF, respectively. Heterogeneous and significant age-dependent rCBF increases were found at specific functional regions of language network during this critical period.
Purpose
Language acquisition and processing are unique cognitive abilities of human beings, and early infancy is a critical period for developing such abilities (1). Specifically, infants start to learn their mother tongue at a remarkably speed, from babbling to full sentences, during age of 6-month to 2-3 years (2). Meanwhile, their brain architectures mature rapidly with dramatic size increases and functional networks emergences (3, 4) to support the behavioral development. Infant regional cerebral blood flow (rCBF), tightly coupled with regional metabolism (e.g. 5, 6), meets the metabolic demands of brain growth. However, the spatiotemporal distribution of rCBF in language network during infancy is not known. In this study, we aimed to identify the language network of infants aged 1-24months and delineate rCBF changes across the functional regions of interests (ROIs) within language network, using multi-model resting-state functional MRI (rs-fMRI) and pseudo-continuous arterial-spin-labeling (pCASL) perfusion MRI.Methods
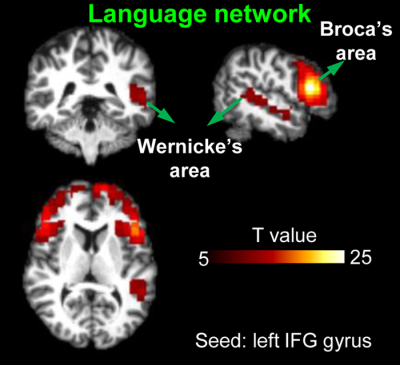
Infant subjects and acquisition of multi-modal MRI: 48 infants (18F/30M, age range: 1 to 24 months) were recruited. Infant MRI images were acquired in a 3T Philips Achieva system. The pCASL perfusion MRI parameters were: multi-slice EPI readout, field of view (FOV) = 230×230 mm2, in-plane resolution = 2.74×2.74 mm2, 20 slices with a thickness of 5mm, no gap between slices, labeling duration = 1650ms, post labeling delay (PLD) = 1600ms, center of labeling slab located at the junction of spinal cord and medulla, TR/TE = 4100/15ms, 30 pairs of controls/labels images. The rs-fMRI parameters were: FOV = 220 x 220 mm2, in-plane imaging resolution = 3.4 x 3.4 mm2, 37 slices with a thickness of 3mm, 1mm gap between slices, TR/TE = 2000/24 ms, 200 dynamics. In addition, both high-resolution T1- and T2-weighted images (T1w and T2w) with a voxel size of 1mm3 were acquired for anatomical guidance. rCBF quantification: rCBF were calculated using the single-compartment model (7): $$$f(x,y,z)=(6000*λ*∆M(x,y,z)*exp(PLD/T_1a ))/(2α∙M_b^0∙T_1a∙(1-exp((-LabelDur)/T_1a )))$$$ where f(x,y,z) is the blood flow at voxel (x,y,z); ∆M(x,y,z) is the difference between dynamic-averaged signal intensity in control image and that in the label image at voxel (x, y, z); labeling efficiency was assumed to be 0.85, and blood T1 value of arterial blood was assumed to be 1800ms (8), $$$M_b^0$$$ values were estimated with the M0 images from the acquisition. Identification of functional ROIs in language network: After preprocessing of rs-fMRI, seed-based connectivity analysis was conducted to generate ROIs of language (seed: left inferior frontal gyrus, IFG) and sensorimotor (seed: left precentral gyrus) networks in a template space (MNI). Here, seed ROIs were defined from previous studies related to language network (9,10) with a sphere of 6mm. Group-level significant functional connectivity (FC) maps were obtained using one-sample T-test with false discovery rate (FDR) correction (p<0.001). The identified functional ROIs in language network include left-IFG and left-anterior/posterior superior temporal gyrus (aSTG/pSTG). Age effect of rCBF at different ROIs in language network: To extract the rCBF values in identified functional networks, infants’ CBF maps were transferred to the template space with combined affine and nonlinear transformations, using the contracts of T1w to drive the registration. Averaged rCBF values at identified ROIs in language and sensorimotor networks were calculated and correlated with subject age using linear regression.Results
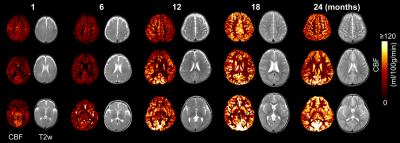
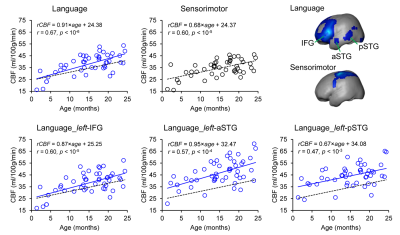
Fig. 1 shows the axial rCBF maps along with corresponding T2w images as anatomical reference from five representative infants at milestone ages of 1, 6, 12, 18, and 24months. The general increase of rCBF across the brain regions can be observed from birth to 2 years of age. To explore the rCBF changes at specific functional regions of the language network during this critical period, a seed-based connectivity analysis was employed to identify the functional ROIs. When seeded in the relevant inferior frontal brain region, significant activation of brain regions known to be involved in language processing, including left inferior frontal (Broca’s area) and superior temporal gyrus/sulcus (Wernicke’s area), are shown in Fig. 2. The identified functional ROIs from language and sensorimotor networks are shown in the upper right panel of Fig. 3. rCBF at these ROIs all increased significantly with age (all p < 10-3). With the rCBF growth trend line of primary sensorimotor network (black dashed lines) in Fig. 3 used as reference, the regional CBF increase are heterogeneous across the subregions of language network. Specifically, rCBF increase rate seems to be higher in the overall language network, subregion of left-IFG and left-aSTG, compared to that in the sensorimotor network.Discussion and conclusion
Regional CBF increased significantly and heterogeneously among functional brain regions in language network from 0 to 2 years. Specifically, rCBF increases with a higher rate at left-IFG and left aSTG, compared to pSTG. Such differential rates may underlie the distinctive metabolic needs that drive the increase of blood supply during infant language brain circuit formation. For instance, left-IFG known as Broca’s area involves in the top-down processes of language network that develop later than the bottom-up processes primary implemented in STG (11). Quantifying rCBF across functional brain regions may offer insight into the physiological aspects of the emergence of certain functional networks. Analyses of diffusion MRI is under way to examine the structural maturation of the language network.Acknowledgements
This study is funded by NIH MH092535, MH092535-S1 and HD086984.References
[1] Werker JF, Hensch TK, 2015. Critical periods in speech perception: new directions. Annt Rev Psychol, 66:173-196.
[2] Kuhl PK, 2004. Early language acquisition: cracking the speech code. Nat Rev Neurosci, 5(11): 831-843.
[3] Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO, 1994. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 51(9): 874-887.
[4] Ouyang M, Dubois J, Yu Q, Mukherjee P, Huang H, 2019. Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage, 185: 836-850.
[5] Fox, P.T. and Raichle, M.E., 1986. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83(4):1140-1144.
[6] Vaishnavi, S.N., Vlassenko, A.G., Rundle, M.M., Snyder, A.Z., Mintun, M.A. and Raichle, M.E., 2010. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA, 107(41): 17757-17762.
[7] Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, et al, 2015. Recommended implementationof arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116.
[8] Varela M, Hajnal JV, Petersen ET, Golay X, Merchant N, Larkman DJ, 2011. A method for rapid in vivo measurement of blood T1. NMR Biomed. 24: 80–88.
[9] Tomasi D, Volkow ND, 2012. Resting functional connectivity of language networks: characterization and reproducibility. Molecular Psychiatry, 17(8):841-854.
[10] Lohmann G, Hoehl S, Brauer J, Danielmeier C, Bornkessel-Schlesewsky I, Bahlmann J, Turner R, Friederici A, 2010. Setting the frame: the human brain activates a basic low-frequency network for language processing. Cerebral Cortex 20(6):1286-1292.
[11] Skeide MA, Friederici AD, 2016. The ontogeny of the cortical language network. Nat Rev Neurosci, 17(5): 323-332.
Figures