4630
Predictive Model of MRI Screening for Neonatal Punctate White Matter Lesion Based on Multi-Center Data1Department of Radiology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi'an, China, 2MR Research China, GE Healthcare, Xi'an, China
Synopsis
PWML with high incidence (~20%) was correlated with adverse neurodevelopment outcomes and lesions disappeared as time goes on. Although MRI can accurately evaluate PWML, currently it’s not a universal screening method. This study aimed to develop a predictive model of MRI screening for PWML based on clinical factors, and help clinicians identify which neonates should undergo MRI. 5899 neonates were recruited. Logistic regression analysis were used to develop the model. Ten independent factors were identified and the model was presented as nomogram. Current model showed good discrimination and clinically use. It will help clinicians conveniently and early make decision.
Introduction
The incidence of punctate white matter lesion (PWML) is as high as 20% 1, 2, and strongly correlates with adverse neurodevelopment outcomes 3, 4. Although magnetic resonance imaging (MRI) can accurately evaluate the degree of PWML and provide rich prognostic information, currently it is not a universal screening method for PWML. PWML are detected in greater numbers and different patterns during the first weeks of life 2, 5. But as time goes on, PWML will change and disappear 6. Due to the age-dependent effects of these lesions, clinicians need efficient methods to identify which neonates should undergo MRI. This study aimed to extract maternal and neonatal clinical features to develop a predictive model of MRI screening for PWML using logistic regression analysis and presenting as nomogram.Materials and Methods
We performed a prospective, observational cohort study between December 2011 and March 2019 at 9 clinical centers. Neonates (n=5899) who underwent MRI examination and had perinatal and postnatal clinical features were recruited. An independent training database comprising 4129 neonates randomly assigned and a validation database of 1770 neonates were created. Neonates were divided into PWML and non-PWML groups. Univariate and multivariate analysis of the logistic regression were used to explore the independent risk factors of PWML, while the different centers were adjusted as a covariate. The model for predicting risk of PWML that incorporated the above independent predictors was developed, validated and presented as the nomogram. Model performance was internally validated using 10-fold cross-validation. Predictive performance was measured by estimating the area under the ROC curve (AUC), calibration curve and decision curve analysis (DCA). Data analysis was performed between March 18, 2019 and September 15, 2019.Results
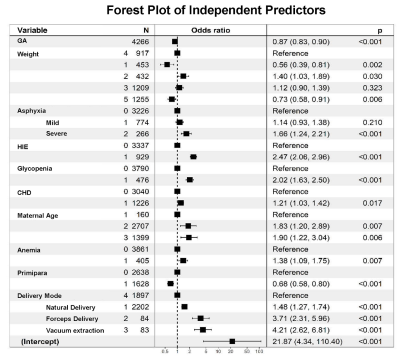
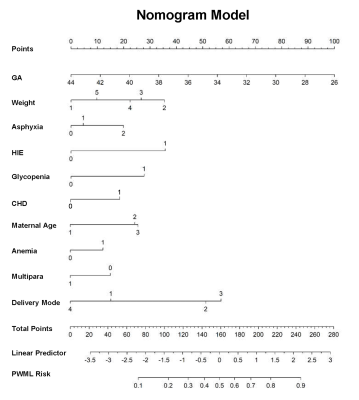
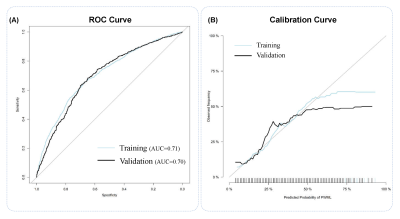
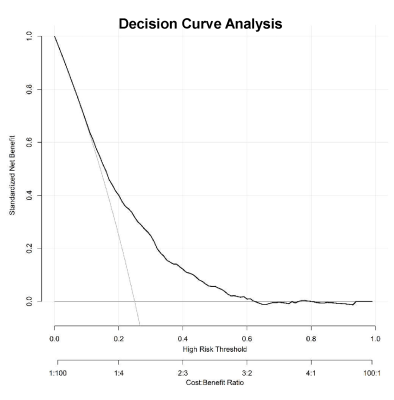
This study recruited 5899 neonates of 9 clinical centers, and the total incidence of PWML was 23.07% (1361/5899).Thirty-seven clinical factors were analyzed by univariate logistic regression, and 26 factors (P<0.1) were included for the further analysis of binary logistic regression. Finally, 10 independent predictors were identified (P<0.05) (Figure 1) and contained in the individualized prediction nomogram model (Figure 2). The model was further tested in the validation dataset. Among the postnatal factors, hypoxic ischemic encephalopathy (HIE), glycopenia obviously increased the risk of PWML with OR 2. 47 (95% CI, 2.06-2.96) and 2.02 (95% CI, 1.63-2.50). Also asphyxia, congenital heart disease, weight were the independent risk factors. The gestational age was protective factor. For perinatal factors, vacuum extraction (OR 4.21; 95% CI 2.62-6.81) had a higher odds of PWML compared with cesarean section. Maternal age, anemia and primiparity were the independent risk factors. The current nomogram model showed good discrimination, with AUC of 0.71 (0.69~0.73), and good calibration. (Figure 3). Application of the nomogram in the validation dataset still gave good discrimination (AUC, 0.70 [0.67 ~ 0.73]) and good calibration(Figure 3). Decision curve analysis demonstrated that the screening nomogram model was clinically useful (Figure 4).Discussion
We developed and validated a predictive nomogram model based clinical features for assisting clinicians to screen neonates with high risk of PWML who should undergo MRI. Ten independent factors were identified for predictive model. Many studies focused only on describing PWML, excluding control neonates without MRI abnormalities and used less sample 2. In this study, we included all neonates who had MRI scan available within 9 centers, providing a unique opportunity to identify risk factors for PWML and establish a screening model. Compared with previous studies 1, 3, 4, 7, we included more perinatal and postnatal factors about 37, such as maternal age, anemia, several maternal diseases, neonatal birth weight, Apgar, neonatal infections and CHD and so on. And PWML have been associated with adverse neurodevelopmental outcomes in several studies4, 8, with the number of lesions negatively correlated with outcome. Our predictive nomogram model was simple rule to predict risk of neonatal PWML and screen neonates who were necessary to undergo MRI. This model was conducive to early and accurately detect lesions.In our study, the incidence of PWML was approximately 23.7%, consistent with other studies 2, 5. For postnatal factors, preterm was found related to PWML, it has been demonstrated by previous studies 9. Greater birth weight, as an independent factors, which was consisted with Wagenaar et al 1. It may be due to that children with a greater birth weight were scanned earlier in life, and the early scanning, the PWML were more prone to be detected on MRI. Asphyxia, HIE, and CHD, glycopenia could induce white matter injury, also observed in other studies10-12. For maternal factors, assisted delivery had a higher odds of PWML compared with cesarean section. It may be related to the increased risk of cerebral ischemia and hypoxia during delivery. Multipara has a lower risk than multipara. It may due to that delivery time of multipara is much shorter than that of primipara, and the ischemia and hypoxia caused by birth canal extrusion may be shorter.Conclusion
The predictive nomogram model estimated the probability of PWML by evaluating the risk factors present in the individual neonates. If implemented, this predictive model may help clinicians conveniently screening neonates of necessary MRI scans, without missing more neonates with PWML based on the age-dependent lesions.Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (81901516, 81971581, 81901823, 81771810 and 51706178), Shaanxi Provincial Innovation Team (2019TD-018), National Key Research and Development Program of China (2016YFC0100300), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438), the Project Funded by China Postdoctoral Science Foundation (No. 2019M653659), and the Natural Science Basic Research Plan in Shaanxi Province of China (No.2019JQ-198).References
1. Wagenaar N, Chau V, Groenendaal F, et al. Clinical Risk Factors for Punctate White Matter Lesions on Early Magnetic Resonance Imaging in Preterm Newborns. J Pediatr 2017;182:34-40 e31
2. Kersbergen KJ, Benders MJ, Groenendaal F, et al. Different patterns of punctate white matter lesions in serially scanned preterm infants. PLoS One 2014;9:e108904
3. Guo T, Duerden EG, Adams E, et al. Quantitative assessment of white matter injury in preterm neonates: Association with outcomes. Neurology 2017;88:614-622
4. Tusor N, Benders MJ, Counsell SJ, et al. Punctate White Matter Lesions Associated With Altered Brain Development And Adverse Motor Outcome In Preterm Infants. Sci Rep 2017;7:13250
5. Martinez-Biarge M, Groenendaal F, Kersbergen KJ, et al. MRI Based Preterm White Matter Injury Classification: The Importance of Sequential Imaging in Determining Severity of Injury. PLoS One 2016;11:e0156245
6. Weeke LC, Brilstra E, Braun KP, et al. Punctate white matter lesions in full-term infants with neonatal seizures associated with SLC13A5 mutations. Eur J Paediatr Neurol 2017;21:396-403
7. Hayman M, van Wezel-Meijler G, van Straaten H, et al. Punctate white-matter lesions in the full-term newborn: Underlying aetiology and outcome. Eur J Paediatr Neurol 2019
8. de Bruine FT, van den Berg-Huysmans AA, Leijser LM, et al. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology 2011;261:899-906
9. Nguyen ALA, Ding Y, Suffren S, et al. The brain’s kryptonite: Overview of punctate white matter lesions in neonates. International Journal of Developmental Neuroscience 2019
10. Swarte R, Lequin M, Cherian P, et al. Imaging patterns of brain injury in term-birth asphyxia. Acta paediatrica (Oslo, Norway : 1992) 2009;98:586-592
11. Guo T, Chau V, Peyvandi S, et al. White matter injury in term neonates with congenital heart diseases: Topology & comparison with preterm newborns. Neuroimage 2019;185:742-749
12. Stomnaroska O, Petkovska E, Jancevska S, et al. Neonatal Hypoglycemia: Risk Factors and Outcomes. Prilozi (Makedonska akademija na naukite i umetnostite Oddelenie za medicinski nauki) 2017;38:97-101