4625
DKI is a sensitive method for detect the white matter patho-morphological variations in neonates with mild white matter injury1The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, 2MR Research China, GE Healthcare, Beijing, China
Synopsis
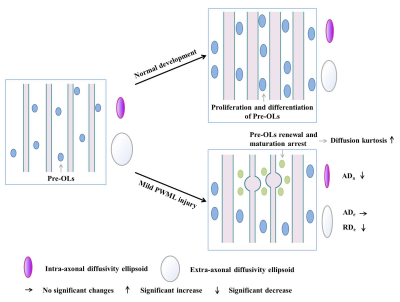
White matter injury is common in neonates. The most common punctate white matter lesions (PWML) can disappear along with time and are easily missed diagnosis. Since DTI is not sensitive to detect the white matter (WM) microstructural changes in mild PWMLs, DKI may provide more details for capture the WM alterations. This study aims to investigate the utility of DKI in characterizing the WM microstructural variations following mild PWMLs. Our study found that diffusion kurtosis and two-compartment metrics are sensitive imaging markers for characterize the WM microstructural alterations of mild PWMLs, which is associated with reactive gliosis and axonal swelling.
Introduction
White matter injury (WMI) is common in neonates, with the most common punctate white matter lesions (PWML) can be detected on conventional MRI as hyperintensity on T1WI and hypointenity on T2WI.1 It is important to note that these lesions can gradually disappear along with time and are easily missed diagnosis, especially for the mild injury with small lesions and variable location.2 The extensive white matter (WM) microstructural abnormalities caused by PWMLs were reported in several DTI studies; however, the value of this method is limited in the mild subgroup.1, 3 Since DKI derived parameters have shown great promise to better capture brain microstructural changes in the developing brain, 4 this study aims to investigate the utility of DKI in characterizing the WM microstructural variations following mild PWMLsMethods
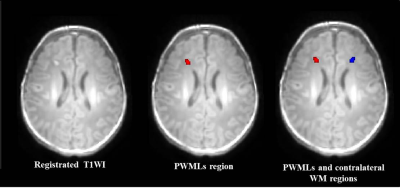
The local institutional review board approved this study and all the written informed consents were obtained from parents of neonates. Subjects Neonates with evidence of mild PWML (diagnosed by conventional MRI: ≤6 lesions or 1 large lesion [diameter≥5mm]) were included. Subjects with symmetrical distribution of lesions were excluded. MRI Protocols All MR examinations were performed using a 3T scanner (Signa HDxt, GE Healthcare, Milwaukee, Wisconsin) with an 8-channel head coil. The parameters of DKI sequence were as follows: TR/TE=8000-10000ms/91.7-126.1ms; the number of b0=4; b values=0, 500, 1000, 2000, 2500 s/mm2; matrix=128×128; section-thickness=4mm with no gap and FOV=180mm. Data and statistical analysis DKI data was processed with the FMRIB software library (FSL, www.fmrib.ox.au.uk/fsl). All T1WI were aligned to the DKI-derived parameter maps individually, and further mapped on to the established local symmetrical neonatal template. After manually segmented the PWMLs, lesions were also aligned to the symmetrical template by the same registration matrix and the lesion volume was calculated. The reference ROIs on contralateral WM of each subject were determined by horizontal reversal with the midline of the brain as the center (Figure 1). Wilcoxon paired tests were used for the differences DKI parameters between PWMLs and the matched reference ROIs. Spearman correlation was used to analysis the relationship between PWMLs volume and DKI parameters. All statistical analysis were performed by using SPSS 19.0 (SPSS, Chicago, IL, USA); P<0.05 was considered as statistically significant difference.Results
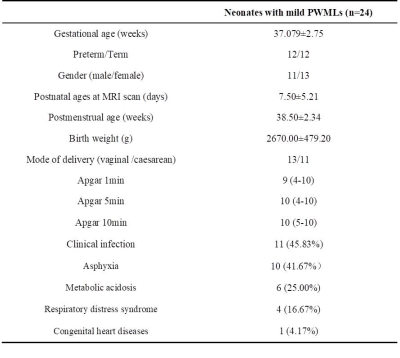
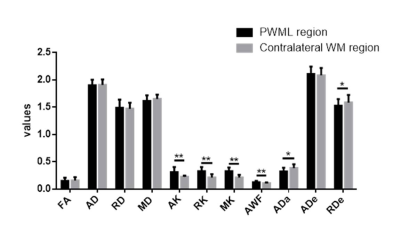
A total of 24 neonates (12 preterm and 12 term subjects, respectively) with mild PWML were enrolled. The demographic information was shown in Table 1. Compared with reference ROIs, the diffusion kurtosis (AK, RK and MK) and axonal water fraction (AWF) were significantly increased in PWML regions, while the intra-axonal axial diffusivity (ADa) and extra-axonal radial diffusivity (RDe) were significantly decreased (P<0.001). No significant differences were found in other parameters, such as FA, AD, RD, MD and extra-axonal axial diffusivity (P>0.005, Figures 2 and 3).Discussion
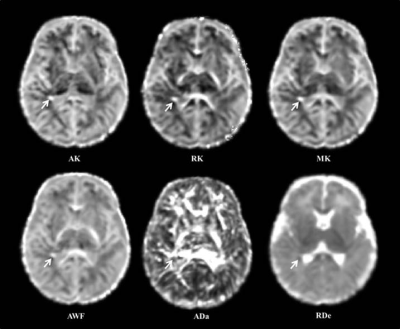
This study quantitatively characterized the WM microstructural alterations in neonates with mild PWMLs, and diffusion kurtosis and two-compartment metrics are sensitive imaging markers for this variations caused by the injury. Diffusion kurtosis is an in vivo measure of the complexity or heterogeneity of the microenvironment in brain tissue.5 The increased diffusion kurtosis may result from the concentrated of dysmaturity premyelinating oligodendrocytes and reactive activated microglia following the injury, which has been proved by the autopsy studies.6, 7 During the neonatal period, most of the WM affected by the injury is in the pre-myelination stage, the aggregation of cells changes the water diffusion outside the axons. Our results revealed that the diffusion radial to the axons is relatively greatly affected by the changes of microenvironment after such damage. Since the PWMLs are not appearing to involve loss of axons, the decreased ADa observed in our study may indicate the axonal swelling, and is consistent with the histopathology in previous study.8 Consistently, the increased intra-axonal water volume and extra-axonal cells finally contribute to the increase of AWF (Figure 4).Conclusion
DKI metrics are sensitive imaging markers for characterize the WM microstructural alterations of mild PWMLs, which is associated with reactive gliosis and axonal swelling.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81971581, 81771810, 81471631 and 51706178), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438) and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF-CRF-2015-004).References
1. Li X, Gao J, Wang M, et al. Characterization of extensive microstructural variations associated with punctate white matter lesions in preterm neonates [J]. American Journal of Neuroradiology, 2017, 38(6): 1228-34.
2. Kersbergen KJ, Benders MJ, Groenendaal F, et al. Different patterns of punctate white matter lesions in serially scanned preterm infants [J]. PloS one, 2014, 9(10): e108904.
3. Bassi L, Chew A, Merchant N, et al. Diffusion tensor imaging in preterm infants with punctate white matter lesions [J]. Pediatric research, 2011, 69(6): 561-6.
4. Paydar A, Fieremans E, Nwankwo JI, et al. Diffusional kurtosis imaging of the developing brain [J]. AJNR American journal of neuroradiology, 2014, 35(4): 808-14.
5. Hui ES, Fieremans E, Jensen JH, et al. Stroke assessment with diffusional kurtosis imaging [J]. Stroke, 2012, 43(11): 2968-73.
6. Rutherford MA, Supramaniam V, Ederies A, et al. Magnetic resonance imaging of white matter diseases of prematurity [J]. Neuroradiology, 2010, 52(6): 505-21.
7. Back SA. White matter injury in the preterm infant: pathology and mechanisms [J]. Acta neuropathologica, 2017, 134(3): 331-49.
8. Back SA, Luo NL, Mallinson RA, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes [J]. Annals of neurology, 2005, 58(1): 108-20.
Figures