4624
Machine-Learning Segmentation and Classification of Pediatric Brain Tumors Based on Preclinical Multiparametric Advanced Fast Imaging (MAFI)1Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 2University of Colorado Anschutz, Aurora, CO, United States, 3Radiology, University of Colorado Anschutz, Denver, CO, United States
Synopsis
Brain tumors are the second most common malignancy in childhood (exceeded only by leukemia). Clinically, multiparametric MRI is now considered to be the neuroimaging standard for detecting brain tumors. Pediatric brain tumors have a diverse array of clinical manifestations, cellular and molecular phenotypes, and tumor habitats. Previously, we reported on the initial development of a non-gadolinium, Multiparametric Advanced Fast Imaging (MAFI) protocol at the 9.4 Tesla in patient derived xenografts models (PDX, ISMRM 2019). Here, we expanded our study to include all existing pediatrics PDX mouse models for machine learning based segmentation and classification of four major brain tumor subtypes.
Introduction
Brain tumors are the second most common malignancy in childhood and clinically, magnetic resonance imaging (MRI) remains a gold standard for detection and follow-up of these malignancies. Moreover, in children, as opposed to in adults, brain tumors are characterized by a more diverse array of clinical manifestations, cellular/molecular phenotypes (1), and tumor habitats. Currently, there is a lack of established human-faithful pediatric brain tumor mouse models and fast high-resolution physiological MRI for their detection and characterization. The goal of this pre-clinical study was to develop a Multiparametric Advanced Fast Imaging (MAFI) approach for rapid and sensitive localization of intracranial tumors and characterization of tumor “habitat” including development of angiogenesis, peritumoral edema and metastatic spread. Patient-derived xenograft (PDX) mouse models for two cerebellar tumors (medulloblastomas and ependymomas) as well as diffuse intrinsic pontine gliomas (DIPG) and atypical teratoid rhabdoid tumors (ATRT, cerebellar, brain stem and spinal cord tumor) were employed in this study (2-4).Methods
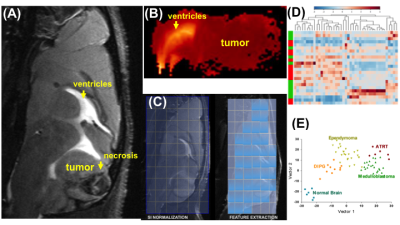
Female immunodeficient (SCID) mice were used for intracranial orthotopical inoculation of pediatric PDX: medulloblastomas (n=13) and ependymomas (n=20) into the fourth ventricle, DPIG (n=8) into the pons, and ATRT (n=6) into the cerebellum. All image acquisition was performed one, three and five weeks after inoculation using an ultra-high field Bruker 9.4 Tesla BioSpec MR scanner and a Bruker mouse head RF array coil. Non-gadolinium MAFI protocols were developed based on high-resolution HR T2w turboRARE (3D, 5min22s), FLAIR (sagittal, 1min54s) and fast spin echo diffusion-weighted imaging (DWI axial, 1min17s). Analytical methodologies included (i) conventional volumetric analysis, blood vessel density and apparent diffusion coefficient (ADC) values using ParaVision360 NEO and PV6.0 software, as well as (ii) shape and texture based radiomics descriptors to characterize tumor habitat of each tumor sub-type using a modified COLIAGE software (5,6). All animal procedures were approved by the institutional IACUC.Results
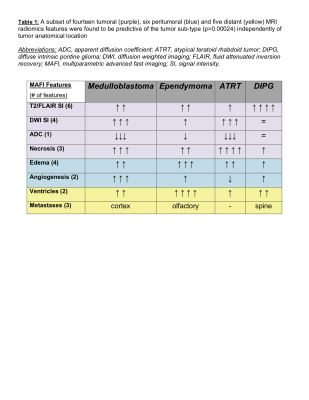
HR T2w-MRI (43 micron in-plane resolution) clearly showed that all PDX were inoculated and homed at the proper anatomical location: the posterior fossa for medulloblastomas; the pons for DIPG; and the cerebellum for ependymomas and ATRT (Figure 1A). The sensitivity of T2w-MRI scans was 0.2 mm3 for the smallest tumor detected. Images also revealed increased blood vessel densities (in the early stage, mean tumor volume 0.2 – 30 mm3), necrosis and intracranial metastases (the late stage, mean tumor volume >40 mm3). FLAIR MRI precisely identified peritumoral regions of the diffuse disease, and together with DWI, of vasogenic edema. The ADC values were notably low in medulloblastomas and ATRT (as low as 0.58x10-3 mm/s) (Figure 1B). For radiomics analysis, each scan was segmented into three regions: (i) well defined tumor (including distant metastases); (ii) peritumoral edema; (iii) tumor necrosis (Figure 1C). 360 radiomics features (capturing co-occurrence, grey-level dependence and directional gradients) were obtained for each region from the MAFI images. A subset of fourteen tumoral, six peritumoral and five distant MRI radiomics features were found to be predictive of the tumor sub-type (p=0.0017) independently of tumor anatomical location (Figure D-E).Discussion
We report that orthotopically implanted PDX xenografts closely mimic histological features, invasiveness, metastasis and radiological features of the primary tumors. Applied MAFI MRI protocol followed by radiomics feature analysis discriminated among specific radiological features for four distinct orthotopic PDX models: medulloblastomas exhibit low ADC values, high angiogenesis and cortical metastases as compared to ependymomas (high levels of edema and olfactory bulb metastases), ATRT (the highest level of necrosis) and DIPG (highest T2 signal intensities and spinal metastases) (Table 1). We further hope to expand our MAFI MRI capacity by performing macrophage-specific T2/T2*-MRI (inflammation) and diffusion-weighted imaging (DWI for edema and necrosis) as reliable predictors for radiation and immunotherapy treatment response in medulloblastomas and high-grade gliomas.Acknowledgements
This works was supported by the St. Baldrick’s Foundation Summer Fellowship Grant Award (Award ID #639631)References
[1] Blumcke I, Spreafico R, et al.: Histopathological findings in brain tissue obtained during epilepsiy surgery. N Engl J Med 2017, 377: 1648-56
[2] Sanden E, Dyberg C, et al.: Establishment and characterization of an orthotopic patient-derived group 3 medulloblastoma model for preclinical drug evaluation. Sci Rep 2017, 7: 46366.
[3] Pierce AM, Keating AK: Creating anatomically accurate and reproducible intracranial xenografts of human brain tumors. J Vis Exp 2014, 24:5, 2017.
[4] Kram DE, Henderson JJ, Baig M, et al. Embryonal Tumors of the Central Nervous System in Children: The Era of Targeted Therapeutics. Bioengineering (Basel). 2018, 5(4):78.
[5] Prasanna P, Patel J, Partovu S, Madabhushi A, Tiwari P: Radiomic features from the peritumoral brain parenchyma on treatment-naïve multi-parametric MR imaging. Eur Radiol 2017, 27: 4188-97.
[6] Beig N, Prasanna P, et al.: Radiogenomic analysis of hypoxia pathway is predictve of overall survival in glioblastoma. Sci Rep 2018, 8: 7.