4621
Size-Adaptable 32-Channel Receive Array for Pediatric Brain Imaging at 3 Tesla
Anpreet Ghotra1, Heather Kosakowski2, Atsushi Takahashi2, Markus May1, Alina Scholz1, Nicolas Kutscha1, Mirsad Mahmutovic1, Lawrence L Wald3, Nancy Kanwisher2, Rebecca Saxe2, and Boris Keil1
1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Science, Giessen, Germany, 2McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Martinos Center for Biomedical Imaging, Dept. of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Science, Giessen, Germany, 2McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Martinos Center for Biomedical Imaging, Dept. of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
The aim of this study is a hardware related approach to increase the subject’s compliance for fMRI with awake infants. An infant-friendly head coil array was developed to improve sensitivity, spatial resolution, accelerated encoding, motion insensitivity, and subject tolerance in pediatric MR-imaging. The coil was characterized with both bench and image metrics and compared to a 32-channel adult head coil. The size-optimized 32-channel infant array coil provides increased reception sensitivity and is well-suited for highly accelerated fMRI data acquisitions in awake infants.
Introduction
Recently, substantial effort has been made to bring fMRI to the pediatric population and it has already given a great insight into the developmental changes that take place after birth across multiple fields of inquiry [1-3]. Applications of fMRI with infants and toddlers became a fast-paced expanding field of research. However, the use of MRI during infancy and toddlerhood remains a challenging undertaking due to practical, methodological, and analytical issues that arise when imaging this young and uncooperative population. The rapid changes in head sizes are a major issue for obtaining optimum signal reception in infant MR brain imaging applications during early life, where a close-fitting nature of the coil helmet design is critical to gaining maximum sensitivity from high-density coil arrays. Therefore, the concept of an adjustable coil array is explored for awake infant brain imaging.Methods
A 32-channel pediatric head coil was developed to accommodate head sizes for infants of 1 to 18 months. We implemented the coil helmet design with three anatomical-shaped independent coil segments (Fig. 1). The posterior part was integrated into the coil base, so that the infant can be easily laid down without any other restrictive coil parts. Instead of lying down flat on the patient’s table, we designed the coil base as an inclined cradle seat to improve the subject’s overall MRI scanning compliance (Fig. 2). A critical design component was the implementation of dedicated earmuff compartments in both right and left anterior coil housing sections. For further head immobilization, we placed inflatable cushions to the outer side of the anterior coil parts.We subdivided the total channel count into the posterior part with 12 elements, while the two anterior head parts houses 10 elements each. 30 loop elements were measured to be approximately 65 mm. Two larger loop elements which surround the earmuff compartments comprise elliptical loop diameters of $$$d_1 = 85$$$ mm and $$$d_2 = 91$$$ mm. Bench measurements verified the element’s $$$Q_U / Q_L$$$-ratio, tuning, active detuning, nearest-neighbor coupling and preamplifier decoupling [6] for each coil element. Pairs of coils were attached to a twin preamplifier.
Initial imaging was carried out on a 3T Scanner (Prisma, Siemens Healthineers, Erlangen, Germany). SNR [7], noise correlation, and G-factor calculations [8,9] were calculated from PD weighted raw-data images and compared to a commercially available 32ch head coil. Initial fMRI scans were conducted on 19 awake infants (3-9 months old) while watching videos of faces, bodies, objects, and scenes. Critically, infants had adequate hearing protection and listened to lullabies on infant-specific, MR-compatible headphones. EPI data were collected with 44 near-axial slices (TR/TE/α=3s/30ms/90°, FOV=160mm, matrix=80x80, SL=2mm).
Results
The coil elements showed a $$$Q_U / Q_L$$$-ratio of 4.3 (ear loops $$$ Q_U / Q_L = 8.6 $$$). The average geometrical decoupling of nearest neighbors was measured to be -(15 ± 3) dB. The coupling between non-adjacent pairs ranged from -11 dB to -26 dB. All decoupling values were further reduced by (17 ± 2) dB via preamplifier decoupling. Active detuning provided >42 dB isolation. The noise correlation between the channels ranged from 0.2% to 38.5%, when the coil housing is adjusted for the smallest head size. The SNR gain (Fig. 3) of the constructed 32ch infant coil in the cortical region was measured to be 3-fold, when compared to the 32ch adult head coil. A 25% SNR improvement was still observed in the center. Figure 4 shows the inverse G-factor for Simultaneous MultiSlice techniques. The SMS G-factor maps show substantial improvements using the infant array coil when compared to the adult head coil. Figure 5 shows data from a single awake infant (6 months old) with 13.5 minutes of low-motion data on two canonical contrasts: faces>scenes and faces>objects.Discussion
Several design considerations have to be addressed for an array coil when imaging this young population. The three-segment design provides a compromise between a high-degree of geometrical adaptability, and it greatly simplifies the handling of the coil setup on the scanner’s patient table. Maintaining a tightly fit of the 32-channels around the subject’s head is critical in gaining SNR. However, it potentially limits the usage of proper ear protection. Therefore, we implemented dedicated compartments in the lateral housing parts. The coil was designed as an inclined seated cradle. According to our infant MRI scan experiences obtained from initial studies [10], the infant favors the seated position over the regular supine position, which is typically used during adult MRI brain examinations.Conclusion
By optimizing the shape and functionality of an infant brain array coil to allow head size adaptability, high-level sound protection, and head motion restriction, we improved infant MRI brain imaging in terms of sensitivity, spatial resolution, and accelerated encoding capabilities. We could demonstrate SNR gains, fast image encoding power, and improved scan completion rates by capitalizing on technical advances for both coil array technology and mechanical features, especially tailored for infant population. The coil is well-suited for awake infant neuro MRI studies.Acknowledgements
MINT Fondation, MITReferences
[1] Konishi Y, et al. Functional Brain Imaging Using fMRI and Optical TopographyinInfancy. Sleep Med 2002 Dec;3 Suppl 2:S41–43. [2] Dinstein I, et al. Disrupted Neural Synchronization in Toddlers with Autism. Neuron 2011 Jun;70(6):1218–1225. [3] Damaraju E, et al. Functional Connectivity in the Developing Brain: A Longitudinal Study from 4 to 9months of Age. Neuroimage 2014 Jan;84:169–180. [4] Hughes EJ, et al. A Dedicated Neonatal Brain Imaging System. Magn Reson Med 2017 Aug;78(2):794–804. [5] Deen B, et al. Organization of High-Level Visual Cortexin Human Infants. Nat Commun 2017 Oct;8:13995. [6] Roemer PB, et al. The NMR Phased Array. Magnetic Resonance in Medicine 1990;16(2):192–225. [7] Kellman P, McVeigh ER. Image Reconstruction in SNR Units: A General Method for SNR Measurement. Magn Reson Med 2005 Dec;54(6):1439–1447. [8] Pruessmann KP, et al. SENSE: Sensitivity Encoding for Fast MRI. MagneticResonance in Medicine 1999;42(5):952–962. [9] Setsompop K, et al. Blipped-Controlled Aliasing in Parallel Imaging for Simultaneous Multislice Echo Planar Imaging with Reduced g-Factor Penalty. Magnetic Resonance in Medicine 2012;67(5):1210–1224. [10] Keil B et al. Size-Optimized 32-Channel Brain Arrays for 3 T Pediatric Imaging. Magn Reson Med 2011 Dec;66(6):1777–1787.Figures

Figure 1: Constructed and populated infant coil array.
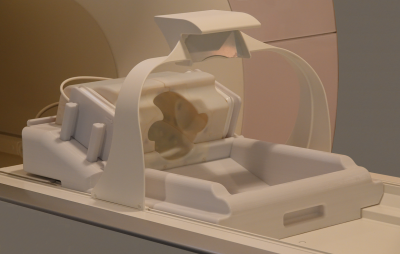
Figure 2: Completed infant array coil installed on the scanner’s patient table.

Figure 3: SNR comparison between the constructed 32-channel infant head coil and the commercial 32-channel adult head coil. The infant coil array shows a 2.7-fold SNR increase across the whole brain region in comparison to the adult head coil. In the peripheral and the central region, the infant coil outperforms the adult coil by 3-fold and 1.25-fold SNR gain, respectively.
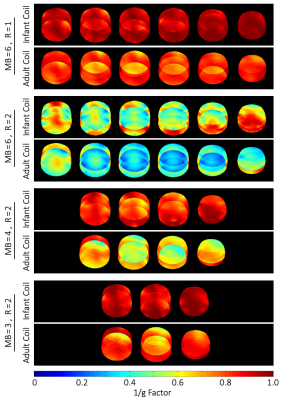
Figure 4: Comparison of accelerated image encoding capabilities when using Simultaneous MultiSlice (SMS) reconstruction techniques. The constructed infant head coil shows substantial lower noise amplification during SMS or combined SMS / in-plane acceleration methods when compared to the adult head coil.
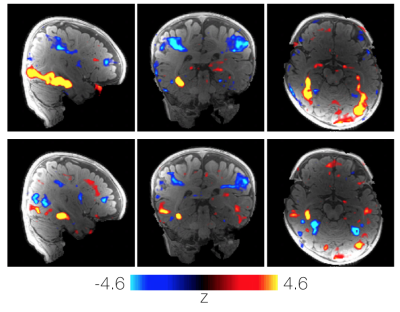
Figure 5: Comparison of two canonical contrast faces>scenes (top) and faces>objects (bottom) in an example subject. Data collected from an awake 6-month-old infant watching colorful videos of faces, bodies, objects and scenes. After motion scrubbing, 13.5 minutes of data was available for inclusion in the analysis. Face activations (red) are in the same areas for both the contrast of faces>scenes (top) and faces>objects (bottom).