4620
An integrated multi‐echo denoising strategy improves the performance of the FPS-fMRI approach on a face localizer1Center for Brain Imaging Science and Technology, Key Laboratory for Biomedical Engineering of Ministry of Education, College of Biomedical Engineering and Instrumental Science, Zhejiang University, Hangzhou, Zhejiang, China, Hangzhou, China, 2Department of Psychology and Behavioral Sciences, Zhejiang University, Hangzhou, China, Hangzhou, China, 3Center for Psychological Sciences, Zhejiang University, China, Hangzhou, China, 4MR Collaboration NE Asia, Siemens Healthcare, Shanghai, China, Shanghai, China, 5Department of Imaging Sciences, University of Rochester, Rochester, NY, USA, Rochester, NY, United States
Synopsis
The fast periodic stimulation (FPS) fMRI approach can be influenced by noise with its frequency spectrum close to the specific task frequency. In this study, we investigated the effect of an integrated multi-echo denoising strategy compared with a single-echo strategy on a face localizer task. The group-averaged SNR increased on a typical face processing network due to a significant decrease in the noise frequency standard deviation. False-positive error introduced by the denoising process could be well tolerated in the presence of a positive activation increase.
Introduction
Fast periodic stimulation (FPS) functional MRI is a novel approach for localizing category-selective neural responses1. In this approach, visual stimuli are presented at a fast presentation rate (6 Hz), and periodic transient switches from the non-target object category to the target category are introduced to modulate neural activity at a specific frequency (target frequency, TF). Target frequency and the neighboring frequency (named noise frequency (NF)) are used to define a signal-to-noise ratio to represent the activation level. fMRI signals are widely affected at full frequency range by motion, cardiac and other factors. The physiological noise frequency spectrum could alias to the much lower frequency bands, causing an overlap with target frequency and noise frequency2, 3, particularly for cardiac-induced noise4. In this study, we proposed to adopt a multi-echo denoising strategy (MEDN) to improve the activation detection with FPS-fMRI.Methods
ParticipantsTwenty-four healthy adult subjects (14 men; 22.2±1.3 years of age) who gave written informed consent participated in this study.
Task design
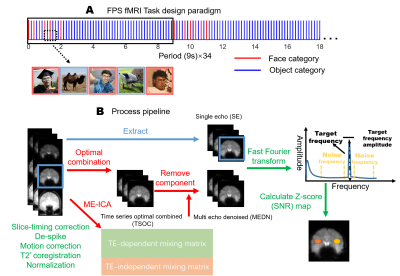
A stimulation paradigm is shown in Figure 1A. Face images and object images alternately presented in the first two seconds of one period; only object images presented for the remaining seven seconds. In total 34 periods were repeated in one run.
MR image acquisition
MR images were acquired using a 3T MAGNETOM Prisma (Siemens Healthcare, Erlangen, Germany) with a 64-channel head coil. Functional images were collected with multi-band, multi-echo, gradient-echo echoplanar imaging (EPI) with the following parameters: TR = 1000 ms; TE = 15, 37.7, 60.4 ms; flip angle = 68°; FOV = 240 × 240 mm2; voxel size = 3 mm isotropic; multi-band accel. factor = 4; GRAPPA = 2; and slices = 44 covering the whole brain. Three runs and additional resting-state fMRI were performed for each subject.
Processing pipeline
The processing pipeline is illustrated in Figure 1B. The MEDN strategy containing an optimal combination and ME-ICA were described by Kundu et. al.5. A z-score (SNR) is defined below: $$SNR=(A_s-μ_N)/σ_N,$$ where $$$A_s$$$ is the TF amplitude, $$$μ_N$$$ is the NF amplitude mean, and $$$σ_N$$$ is the NF amplitude standard deviation (SD). Noise frequency is defined as 40 neighboring frequency bins (20 on each side), as shown in Figure 1B. More details are described by Gao et. al.1.
Results
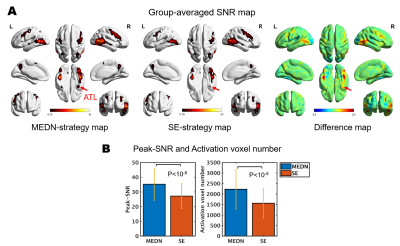
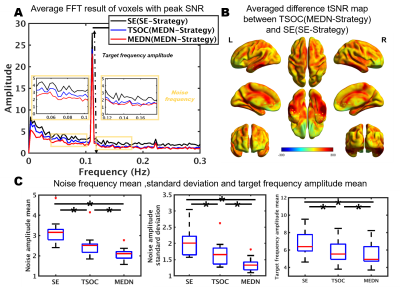
Group-averaged SNR maps of the MEDN strategy and SE strategy are shown in Figure 2. A typical face processing network6 in the lateral fusiform gyrus (FG), inferior occipital gyrus (IOG), and posterior superior temporal sulcus (pSTS) was identified in both strategies. In the MEDN strategy, significant activations were found in the anterior temporal lobe (ATL). The SNR (Z-score) significantly increased in the FG and pSTS. The peak SNR (p < 10-8) and activation voxel number (p < 10-8) in the whole brain also increased.The group-averaged FFT amplitude spectrum was acquired from voxels with a peak SNR of the MEDN strategy and SE strategy. A time series with optimal combination (TSOC)7 result of the MEDN strategy is also presented in Figure 3A. Various measures of effects of the MEDN strategy are shown in Figure 3C. Compared with the TSOC result, the amplitude of the NF bins on the low-frequency side decreased while the other NF bins on the high-frequency side had less of a decrease.
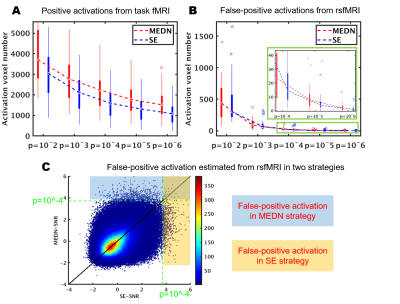
False-positive activation can be estimated using resting-state fMRI (rsfMRI) data8. This activation widely exists under low activation threshold levels, as shown in Figure 4B. The MEDN strategy brought more false-positive activation at all activation threshold levels. However, the false-positive activation increase introduced by the MEDN strategy was much less than the positive activation increase in the task fMRI. The SNR calculated from the rsfMRI data in the two strategies in Figure 4C showed that most brain voxels activate in only one condition.
Discussion
The MEDN strategy significantly improved the performance of the FPS-fMRI approach in the group-averaged results. Both the optimal combination and ME-ICA denoising process decreased the noise frequency amplitude SD, and ME-ICA denoising primarily impacted lower frequency bands. We hypothesized that physiological noise contributes more energy on low-frequency bands and ME-ICA denoising, primarily by removing physiological noise.With an under-sampling rate of 1 Hz (TR = 1 s), typical cardiac noise at the primary frequency is aliased into the low-frequency range. While the target frequency is 1/9 Hz, it is necessary to consider the impact of cardiac noise on the calculation of the SNR. ME-ICA denoising is an excellent tool for removing cardiac noise because it primarily influences BOLD contrast on the alteration of S0. The cardiac component decomposed by ICA can be identified and removed through TE-dependency analysis. The integrated multi-echo denoising strategy not only improves the SNR of the typical face process network but also helps identify the activations in ATL, which have rarely been found in fMRI studies due to magnetic susceptibility artifacts9.
Conclusion
An effective denoising strategy should be introduced in the FPS-fMRI approach to reduce the influence of noise with a frequency spectrum close to the target frequency. The ME-ICA denoising approach works well, as shown in this study.Acknowledgements
No acknowledgement found.References
1. Gao, X., F. Gentile, and B. Rossion, Fast periodic stimulation (FPS): a highly effective approach in fMRI brain mapping. Brain Structure and Function, 2018. 223(5): p. 2433-2454.
2. Glover, G.H., T.Q. Li, and D. Ress, Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine, 2000. 44(1): p. 162-167.
3. Tong, Y.J., L.M. Hocke, and B.B. Frederick, Low Frequency Systemic Hemodynamic "Noise" in Resting State BOLD fMRI: Characteristics, Causes, Implications, Mitigation Strategies, and Applications. Frontiers in Neuroscience, 2019. 13: p. 18.
4. Huotari, N., et al., Sampling Rate Effects on Resting State fMRI Metrics. Frontiers in Neuroscience, 2019. 13: p. 16.
5. Kundu, P., et al., Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences of the United States of America, 2013. 110(40): p. 16187-16192.
6. Duchaine, B. and G. Yovel, A Revised Neural Framework for Face Processing, in Annual Review of Vision Science, Vol 1, J.A. Movshon and B.A. Wandell, Editors. 2015, Annual Reviews: Palo Alto. p. 393-416.
7. Posse, S., et al., Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magnetic Resonance in Medicine, 1999. 42(1): p. 87-97.
8. Eklund, A., et al., Does parametric fMRI analysis with SPM yield valid results?-An empirical study of 1484 rest datasets. Neuroimage, 2012. 61(3): p. 565-578.
9. Collins, J.A., J.E. Koski, and I.R. Olson, More Than Meets the Eye: The Merging of Perceptual and Conceptual Knowledge in the Anterior Temporal Face Area. Frontiers in Human Neuroscience, 2016. 10: p. 11.
Figures