4619
Application of phase-based motion outlier detection to infant dMRI1Department of Radiology and Imaging Sciences, Indiana University, Indianapolis, IN, United States, 2Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 3Department of Electrical and Computer Engineering, Johns Hopkins University, Baltimore, MD, United States
Synopsis
Detecting and eliminating motion-corrupted slices is crucial in diffusion MRI (dMRI), and particularly essential in imaging neonates. Conventional magnitude-based outlier rejection methods are intensity-based and can usually detect and correct intra-volume movement but can miss outliers in cases of small continuous motions. Phase-based methods can be used to detect motion independently, regardless of the slice-to-volume location. The phase-based method is reasonably accurate and computationally fast, and may be better suited for real-time detection of motion in dMRI. Combining magnitude and phase methods could produce the best results. Here, we evaluate the phase-based method versus the magnitude-based method in neonatal data.
Introduction
Neonates and infants have a greater degree of overall motion compared to adults during MRI. In addition, neonatal and infant dMRI is also different compared to adults dMRI because of the smaller head size and immature, incompletely myelinated white matter tracts. This causes a decrease in the signal to noise ratio, especially at higher b-values. Head motion due to involuntary startle motion of neonates can lead to inaccurate dMRI reconstructions. Therefore, identification of motion in dMRI is a key element to appropriately apply motion correction techniques. The state-of-the-art magnitude-based method (eddy)1,2 is used to identify and correct for motion and current-induced distortions. While DW-magnitude images could identify data corruption as regions of signal drop-outs, DW-phase images may have higher sensitivity for detecting subtle subject motion. DW-phase images may also have higher sensitivity for detecting continuous motion as in neonatal dMRI data. We previously presented a phase-based method of DWI motion detection3 based on Haralick’s textural features4. The Phase Image Texture Analysis for Motion Detection in Diffusion MRI (PITA-MDD) method5 can reliably detect motion in DW-data and was validated on volunteers who moved during dMRI (b-value of 1000 s/mm2). Here, we demonstrate the use of PITA-MDD with neonatal dMRI data acquired at two b-values (1000 and 2000 s/mm2). Although dMRI in infants was performed in a research setting, the scan protocol was similar to what is routinely performed clinically, i.e. imaging during natural sleep without anesthesia. We use the colored FA maps to qualitatively compare the results of (1) applying the magnitude-based correction, (2) applying the phase-based correction, and (3) applying both correction methods fused together.Materials and Methods
Five term-born infants (3M, 2F) were recruited from a prospective observational cohort study in an IRB-approved protocol imaged at age 43 (±7) days. Infants were scanned during natural sleep after feeding. The infants were placed in a vacuum-molding cushion (MedVac, CFI, US) to reduce movement. Neonatal dMRI data were acquired on a Siemens Prisma MAGNETOM scanner (Erlangen, Germany) with a 64 channel head coil using a single-shot spin-echo EPI technique with a multiband factor of 3, TE=72.4 ms, TR=2710 ms, 160 mm field of view, 66 slices and 3:10 (min:sec) acquisition time. Diffusion parameters: a monopolar diffusion scheme was used with one non-diffusion weighted volume and 64 diffusion gradient directions. The resolution in all the cases was 1.5x1.5x1.5 mm3. The b-value=1000 s/mm2 was used in cases of neonate subjects S1 and S2, while a b-value= 2000 s/mm2 was applied with neonate subject S3. Two other neonate datasets with no perceived motion (one with b-value=1000 s/mm2 and another with b-value=2000 s/mm2) were used as examples of the no-motion case. We used motion estimation6,7 to confirm that the motion during the no-motion case was not more than 1.3 mm. The PITA-MDD method detects motion by computing the gray level co-occurrence matrix (GLCM) of the phase image, which we denote by p, and is defined as the distribution of co-occurring values at a given spatial offset4. Four GLCMs were computed using pixel offsets in the four different directions of adjacencies (horizontal, vertical, and left and right diagonals) and were averaged to obtain a final co-occurrence matrix. We then extracted Haralick’s homogeneity index (HHI) for each DW-phase slice from the resulting GLCM as follows,$${HHI={\sum_{i,j} \frac{p(i,j)}{1+|{i-j}|}}}\qquad \qquad \qquad \qquad (1)$$
, where $$${p(i,j)}$$$ denotes element $$${(i,j)}$$$ in the GLCM matrix.
In DW-data with b-value=1000 s/mm2, any slice with HHI < 0.6 was eliminated from the DTI metrics calculations. Whereas in DW-data with b-value=2000 s/mm2, any slice with HHI < 0.5 was eliminated from the DTI metrics calculations. We applied the magnitude-based method to the corresponding DW-magnitude images for comparison. Finally, we combined the two methods by applying the phase-based method to the output of the magnitude-based method.
Results
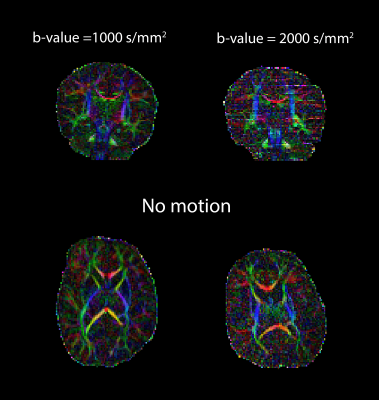
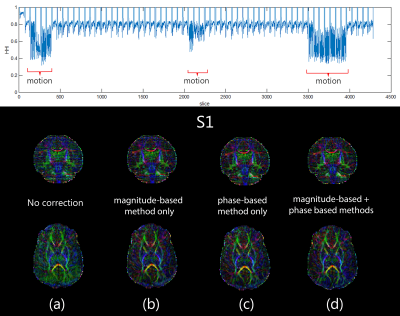
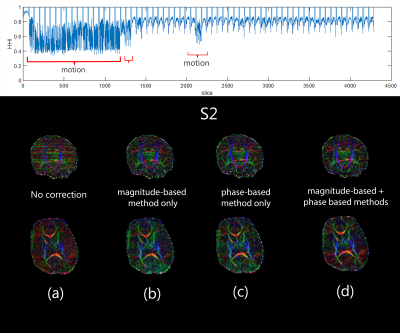
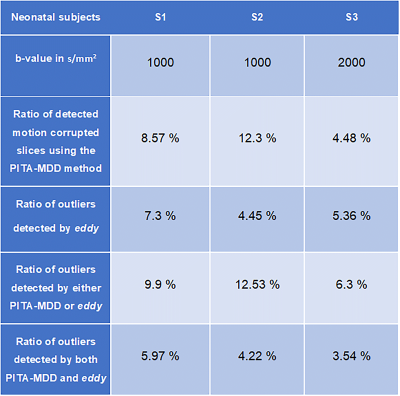
Figure 1 shows an example of the no-motion case of the neonatal colored FA in two b-values (1000 and 2000 s/mm2). Figures 2-4 (top) show the HHI extracted from the DW-phase images of the three neonatal subjects (S1, S2, and S3, respectively). Figures 2-4 (bottom) compare the colored FA maps (a) when the no motion correction method is applied, (b) when the magnitude-based method is applied, (c) when the phase-based method is applied and (d) when applying the two methods fused together. The results of the two methods combined significantly improve the results of each method when used alone. Table 1 shows that the phase-based method detected most of the motion corrupted slices identified by the magnitude-based method. However, there are some slices detected exclusively by each of the two methods.Discussion and Conclusion
Removing motion degraded slices detected by the phase-based method is comparable to using the magnitude-based method alone with the advantage of being computationally fast (2.15 ms/slice). This makes the phase-based method to be more suitable for real-time detection of motion in the case of neonatal/infant dMRI.We also show that combining phase and magnitude-based methods can have qualitatively superior diffusion maps compared to using either method alone. Finally, the phase-based method could be superior to the magnitude-based methods at high b-values that are usually characterized by low signal to noise ratios.
Acknowledgements
This work is supported by the 2018 RSNA seed grant and the 2018 ARRS Scholarship.References
1. Andersson, J. & Sotiropoulos, S., An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063-1078 (2016).
2. Andersson, J., Graham, M., Zsoldos, E. & Sotiropoulos, S., Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage 141, 556-572 (2016).
3. Elsaid, N. et al., Phase-based motion detection for diffusion magnetic resonance imaging, presented at ISMRM, Honolulu, Hawaii, USA, 2017 (unpublished).
4. Haralick, R., Shanmugam, K. & Dinstein, I., Textural features for image classification. IEEE Trans. Systems Man Cybernet. 3, 610–621 (1973).
5. Elsaid, M., Prince, J. L., Roys, S., Gullapalli, R. P. & Zhuo, Phase Image Texture Analysis for Motion Detection in Diffusion MRI (PITA-MDD). Magnetic Resonance Imaging 62, 228-241 (2019).
6. Jenkinson, M. & Smith, S. M., A global optimisation method for robust affine registration of brain images. Medical Image Analysis 5 (2), 143-156 (2001).
7. Jenkinson, M., Bannister, P. R., Brady, J. M. & Smith, S. M., Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17 (2), 825-841 (2002).
Figures