4616
SAR and temperature in neonate models resulting from exposure to 7T head coil1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
A safety study for neonatal MRI at 7T using an generic head transmit coil was performed using two baby voxel models, one of which was created as part of this study. Both adult and age-adjusted tissue properties were used in performing RF simulations. Simulations showed that SAR/W is increased for neonatal imaging, but the efficiency per B1+2 is comparable to adult imaging. The new neonate model additionally had a 'blanket' layer that could be used to simulate thermal insulation as is often used in practice. Thermal simulations suggest that scanning within IEC SAR limits does not lead to excessive heating.
Introduction
The effect of age adjusted dielectric properties, posture, and thermal insulation on SAR and temperature are investigated for neonates positioned in a generic 7T head coil.Methods
Neonate A, created from Dixon MRI images of a 38 week gestational age neonate with a typical posture adopted by neonates undergoing an MR examination, was segmented into 13 tissue types and 0.98x0.98x1.50 mm3 voxels. A ‘blanket’ layer of adjustable properties which covered the body except for the face (Fig 1) was included. Neonate B, based on post mortem CT data of an 8 week old child1,2 and segmented into 31 tissue types, was modified by anisotropic scaling to create a model more representative of the term infant3. A simplified version of Neonate B with the 13 tissue types of Neonate A plus connective tissue was also considered. Each neonate was positioned brain centred within a generic 7T, 16 rung, shielded high pass birdcage head coil similar to that previously described4,5 (Fig 1) placed centrally within a cylindrical shell representing the bore of the scanner. The coil was driven in quadrature. Dielectric properties representative of the high water content of neonatal tissues were calculated following approaches previously reported6,7. Adult tissue properties were taken from Hasgall et al8. Sim4Life v5.0.0 was used for all simulations.Temperature distributions were simulated for Neonate A for exposures at local SAR10g max 10W/kg and SAR head average max 3.2W/kg. In the absence of well established neonatal thermal properties, adult properties were assumed. Since neonates rapidly become hypothermic when left naked at room temperature, mixed thermal boundary conditions (Troom=22°C, h=10W/m2/°C and heat flux=3W/m2) were set at the interface between background and neonate such that its core temperature remained stable with a blanket present but dropped without it.
Results
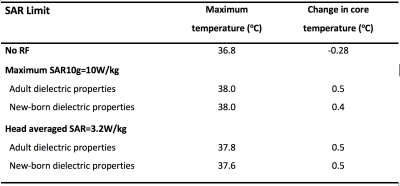
Table 1 shows SAR and mid-slice-averaged $$$B_1^+$$$ for Neonates A and B with different tissue properties, and for adult male "Duke" as a reference. In most cases, the maximum SAR10g occurred in the neck and shoulder region, except for Neonate A with newborn properties when it was in the right hand (Fig 2). Thermal simulations of the naked Neonate A predicted moderate hypothermia (core temperature 35.5°C, $$$\Delta$$$Tcore -2°C) after 20 minutes without RF and typically maximum temperatures ~37°C with 20 minutes exposure at SAR limits. Temperatures predicted for the insulated neonate are shown in Table 2. Maximum temperatures occurred in the skin in the neck and shoulder region.Discussion
SAR normalised to input power is higher in neonates than adults by approximately a factor 2. However when $$$B_1^+$$$ is also considered the values are more similar to adults, suggesting that commonly run sequences will still be possible to use safely.Previous studies of child models for 7T found maximum SAR10g in the heads of several child models (minimum age 5) ranged from 1.85 to 2.3 W/kg/$$$\mu$$$T2. Wigers et al10 reported head average SAR of 0.29-0.47 W/kg/$$$\mu\,T^2$$$ and maximum SAR10g ~1.5-3.0 W/kg/$$$\mu\,T^2$$$ from simulations involving neonate model Charlie11 in a similar coil. Neonate B is derived from the same source as Charlie and our study found comparable maximum SAR10g but with higher head average (0.8W/kg/$$$\mu\,T^2$$$).
The use of age adjusted dielectric properties reflecting the higher water content of neonatal tissues causes the predicted normalised SAR (i.e. per $$$\mu\,T^2$$$) to increase by ~40% largely due to the reduction in mean $$$B_1^+$$$ for the same input power. Neonate model A used a cruder segmentation of tissues (13 types) but comparison with Neonate B suggests this had little effect. The more natural pose of Neonate A with hands close to face and chest resulted in local SAR hot spots in these regions and when newborn properties were used the peak SAR10g occurred in the right hand.
Thermal simulations used boundary conditions that essentially achieved thermal balance when the blanket was present but resulted in moderate hypothermia in its absence. With the blanket, continuous exposures at SAR limits for 20 minutes resulted in local temperature maxima ≤38°C and changes in core temperature≤0.5°C, in compliance with limits12. Thermal modelling of neonates is complex due to their underdeveloped thermal regulation ability, possible use of sedation, high surface to volume ratio - hence high dependence on thermal boundary conditions, and poorly defined tissue thermal properties. The simulations presented here are an initial simple approach to investigating this problem and should be interpreted with caution. There is a risk of neonates becoming hypothermic whilst in the MR environment13. Plaisier et al14 reported that the core temperature of 17% of preterm infants undergoing an MRI procedure at 1.5T was < 36°C and that temperature dropped significantly after the MRI scan, hence our inclusion of the blanket in simulations.
Conclusions
This study aims to establish safety parameters necessary for neonatal imaging using a generic 7T head coil. Maximum SAR per input power is higher than an adult, but this is offset by lower power needed to achieve required $$$B_1^+$$$; however the increased water content of neonatal tissue should also be considered. Ultimately temperature is the key limiting factor: initial thermal simulations are encouraging even if thermal insulation (i.e. blankets etc) are included, however work on accurately modelling the unique thermal conditions for neonates is ongoing.Acknowledgements
This work was supported by ERC grant agreement no. 319456 (dHCP project), the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z) and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1.Veit R, Williams G, Drexler G, et al. Tomographic Anthropomorphic Models, Part I: Construction technique and description of models of an 8 week old Baby and a 7 year old child. Gesellschaft für Strahlen und Umweltforschung: Munich, Germany, 1989, p. 58.
2.Zankl M, Veit R, Williams Get al.. The construction of computer tomographic phantoms and their application in radiology and radiation protection. Radiat. Environ. Biophys. 1988; 27: 153–164.
3.Malik SJ, Beqiri A, Price AN, et al. Specific absorption rate in neonates undergoing magnetic resonance procedures at 1.5 T and 3 T. NMR Biomed. 2015;28:344–352.
4.Restivo MC, van den Berg CAT, van Lier ALHMW, et al. Local specific absorption rate in brain tumors at 7 Tesla. Magn Reson Med 2016;75:381–389.
5.van Lier ALHMW, Kotte ANTJ, Raaymakers BW, et al. Radiofrequency heating induced by 7T head MRI: Thermal assessment using discrete vasculature or Pennes’ Bioheat Equation. J Magn Reson Imaging 2012; 35:795–803.
6.Dimbylow P, Bolch W, Lee C. SAR calculations from 20 MHz to 6 GHz in the University of Florida newborn voxel phantom and their implications for dosimetry. Phys. Med. Biol. 2010;55:1519-150.
7.Peyman A, Rezazadeh AA, Gabriel C. Changes in the dielectric properties of rat tissue as a function of age at microwave frequencies. Phys. Med. Biol. 2001;46:1617-1629.
8. Hasgall PA, Di Gennaro F, Baumgartner C, et al., “IT’IS Database for thermal and electromagnetic parameters of biological tissues,” Version 4.0, May 15, 2018, DOI: 10.13099/VIP21000-04-0.
9. Tiberi G, Fontana N, Costagli M, et al. ,Investigation of maximum local Specific Absorption Rate in 7 T Magnetic Resonance with respect to load size by use of electromagnetic simulations Bioelectromagnetics 2015;36:358-366.
10. Wiegers E, Versteeg E, Hoogduin H, et al. RF safety evaluation of neonatal MRI at 7 T Proc ISMRM Workshop Ultrahigh Field Magnetic Resonance, Dubrovnik, Croatia 31 March-3 April 2019.
11. https://itis.swiss/virtual-population/virtual-population/vip3/charlie/
12. International Electrotechnical Commission (IEC) 60601‐2‐33. Medical Electrical Equipment – Particular Requirements for the Safety of Magnetic Resonance Equipment for Medical Diagnosis. Geneva, 2010.
13. Arthurs OJ, Edwards A, Austin T, et al. The challenges of neonatal magnetic resonance imaging Pediatr Radiol 2012;42:1183-1194.
14. Plaisier A,. Raets MMA, van der Starre C et al. Safety of routine early MRI in preterm infants. Pediatr Radiol 2012;42:1205–1211
Figures

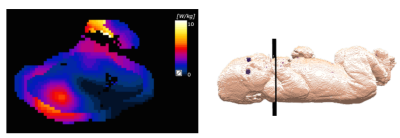
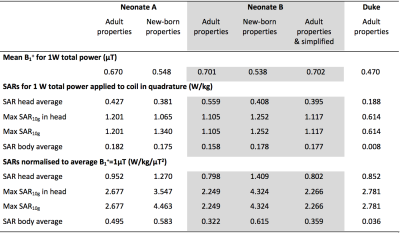
Table 1. Mean B1+ and SARs for 1W total applied power and SARs normalised to mean $$$B_1^+ = 1\mu\,T$$$. Neonate models are run both with 'adult' and also 'new-born' dielectric properties, the latter are adjusted for the higher water content of neonatal tissue. Additionally, data for 'Duke' (adult male) are included for reference. Note the large discrepancy in whole-body SAR between Duke and the neonates, illustrating that the former has only a small exposure area in the head coil, compared with the neonates who are exposed over the majority of their body.