4613
Quantitative assessment of the distribution of mild white matter injury in preterm and term neonates1The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, 2MR Research China, GE Healthcare, Beijing, China
Synopsis
White matter injury is common in neonates. The most common punctate white matter lesions (PWML) can disappear along with time and are easily missed diagnosis. Most current clinical scores account for the numbers and the size of PWML, but ignore the lesion location, which is important for the outcome prediction. This study aims to quantitatively assess the distribution of mild PWML in preterm and term neonates, and characterize its dynamic alterations with gestational age. Our study found that mild PWML is mainly located in the posterior of brain, with extensive injury in the preterm extending to the central region.
Introduction
White matter (WM) injury is common in neonates, with the most common punctate white matter lesions (PWML) is most readily diagnosed on T1WI acquired early in life as areas of hyperintensity.1, 2 These lesions can gradually disappear along with time and are easily missed diagnosis, especially for the mild injury with small lesions and variable location.3 Neuroimaging studies demonstrated that PWML is accompanied by delayed functional development in cognitive and motor outcomes, and the adverse prognosis is associated with the lesion location.2, 4 However, most of the current clinical scores account for the numbers and the size of PWML by visual inspection, and the specific location of lesions are ignored.5, 6 Therefore, this study aims to quantitatively assess the distribution of mild PWML in preterm and term neonates, and characterize its dynamic alterations with gestational age.Methods
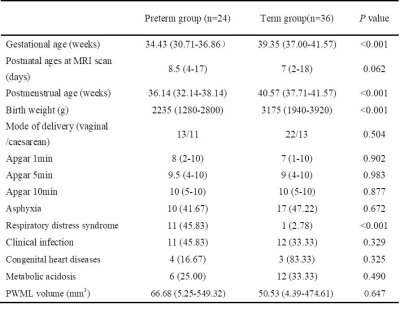
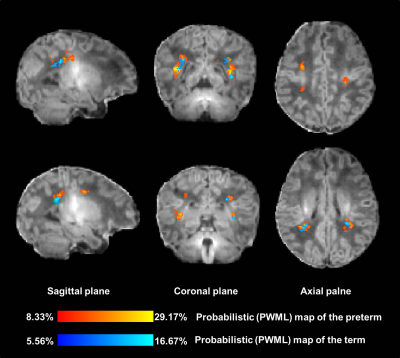
The local institutional review board approved this study and all the written informed consents were obtained from parents of neonates. Subjects Preterm and term neonates with evidence of mild PWML (diagnosed by conventional MRI: ≤6 lesions or 1 large lesion [diameter≥5mm]) were included. Subjects with obvious imaging artifacts were excluded. MRI Protocols All MR examinations were performed using a 3T scanner (Signa HDxt, GE Healthcare, Milwaukee, Wisconsin) with an 8-channel head coil. The parameters of transverse 3D-T1WI sequence were as follows: TR/TE=10ms/4.6ms; matrix=256×256; section-thickness=1mm with no gap; and FOV= 240mm. Data and statistical analysis T1WI data was pre-processed by a multi-view pyramid network.7 The images of all subjects were registered to the JHU-T1WI neonatal template using a combination of linear and non-linear methods. The manually labeled PWML of preterm and term group were mapped to the common space of the template by the same registration matrix, respectively. The probabilistic PWML map of each group was then generated based on the cumulative number of lesions that occurred in homologous brain regions across participant in the standard template (Figure 1). Finally, the probabilistic PWML maps of two groups were both overlaid on the T1WI template to shown the common and different spatial location. Wilcoxon singed-rank and chi-square tests were used to assess group differences in demographics and lesion volume. All statistical analysis were performed by using SPSS 19.0 (SPSS, Chicago, IL, USA); P<0.05 was considered as statistically significant difference.Results
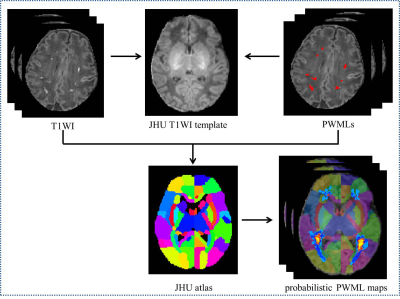
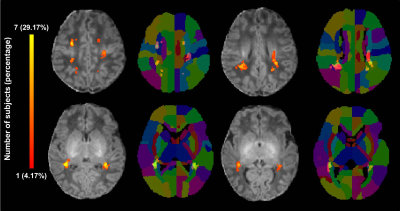
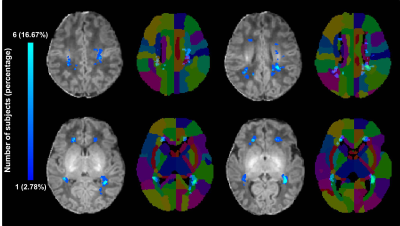
A total of 24 preterm and 36 term neonates with mild PWML were enrolled, respectively. The gestational age, and birth weight were significantly smaller in preterm group than those in the term group (P<0.05). No significant differences in other demographics were found between groups (P>0.05). The incidence of respiratory distress syndrome was significantly higher in the preterm group than that in the term group (P<0.001). No significant difference was observed in lesion volume between the groups (P>0.05, Table 1). To summary the distribution characteristics, PWML occurred at a homologous region in 3 or more neonates in each group were recorded. The PWML probabilistic maps indicated that lesions were distributed in periventricular WM regions, including right posterior thalamic radiation (PTR, 29.17%), left PTR (20.83%), right superior longitudinal fasciculus (20.83%), right supramarginal gyrus (SMG,16.67%), right superior corona radiate (16.67%), left posterior corona radiate (PCR,16.67%), bilateral angular gyrus (AG,16.67%), left postcentral gyrus (12.50%), left SMG (12.50%) and right superior temporal gyrus (12.50%, Figure 2). For term neonates, lesions were mainly located in the watershed areas, including right PCR (16.67%), left PTR (13.89%), bilateral AG (13.89%), right SMG (8.33%), right inferior frontal gyrus (8.33%) and right anterior corona radiate (8.33%, Figure 3). The homologous regions of PWML in preterm and term neonates mainly include bilateral PTR, bilateral AG and right SMG. The overlaid probabilistic map (a homologous region in 2 or more neonates in each group were displayed) revealed that the preterm have relative extensive injury and the lesions are more extended to the central region (Figure 4).Discussion
This study quantitatively assesses the homologous and different lesion distribution in preterm and term neonates with mild PWML. The different injury patterns may cause by a combination of vascular development and cellular susceptibility. In preterm brain, ventriculopetal penetrating arteries extend inward from the surface of the brain to supply the periventricular regions. With the brain development, vessels extend from the lateral ventricles and the intervascular border zone moves peripherally. Therefore, oxidative stress and/or excitotoxicity specifically targets oligodendrocyte precursors in the border zone result in the heterogeneous distribution patterns of PWML between groups.8 For the oligodendrocytes migration, the dorsal wave is the last migration oligodendrocytes and it is start at preterm period (23-32 weeks). Since the dorsal oligodendrocytes show a higher susceptibility to impairments, it can explain the homologous regions of PWML in posterior of the brain.9 Moreover, there are also oligodendrocytes migrate tangentially from ventral to dorsal, and these cells are transforming to radial migration at 28-34 weeks. This tangential-to-radial fiber architectonic is susceptibility to damage within the transformed developmental window, which may be a possible reason for the more extensive injuries of the preterm.10, 11Conclusion
The mild PWML is mainly located in the posterior part of the brain, with extensive injury in the preterm extending to the central region.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81971581, 81771810, 81471631 and 51706178), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438) and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF-CRF-2015-004).References
1. Li X, Gao J, Wang M, et al. Characterization of extensive microstructural variations associated with punctate white matter lesions in preterm neonates [J]. American Journal of Neuroradiology, 2017, 38(6): 1228-34.
2. Guo T, Duerden EG, Adams E, et al. Quantitative assessment of white matter injury in preterm neonates: Association with outcomes [J]. Neurology, 2017, 88(7): 614-22.
3. Kersbergen KJ, Benders MJ, Groenendaal F, et al. Different patterns of punctate white matter lesions in serially scanned preterm infants [J]. PloS one, 2014, 9(10): e108904.
4. Cayam-Rand D, Guo T, Grunau RE, et al. Predicting developmental outcomes in preterm infants: A simple white matter injury imaging rule [J]. Neurology, 2019, 93(13): e1231-e40.
5. Childs AM, Cornette L, Ramenghi LA, et al. Magnetic resonance and cranial ultrasound characteristics of periventricular white matter abnormalities in newborn infants [J]. Clinical radiology, 2001, 56(8): 647-55.
6. Martinez-Biarge M, Groenendaal F, Kersbergen KJ, et al. MRI Based Preterm White Matter Injury Classification: The Importance of Sequential Imaging in Determining Severity of Injury [J]. PloS one, 2016, 11(6): e0156245.
7. Gao Y, Li J, Xu H, et al. A multi-view pyramid network for skull stripping on neonatal T1-weighted MRI [J]. Magnetic resonance imaging, 2019, 63(70-9.
8. Chao CP, Zaleski CG, Patton AC. Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings [J]. Radiographics : a review publication of the Radiological Society of North America, Inc, 2006, 26 Suppl 1(S159-72.
9. van Tilborg E, de Theije CGM, van Hal M, et al. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury [J]. 2018, 66(2): 221-38.
10. Judas M, Rados M, Jovanov-Milosevic N, et al. Structural, immunocytochemical, and mr imaging properties of periventricular crossroads of growing cortical pathways in preterm infants [J]. AJNR American journal of neuroradiology, 2005, 26(10): 2671-84.
11. Marin O, Rubenstein JL. Cell migration in the forebrain [J]. Annual review of neuroscience, 2003, 26: 441-83.
Figures