4612
Decreased fractional anisotropy in the diffusion tensor imaging of neonatal hypoxic ischemic encephalopathy with normal conventional MRI1School of Computer and Control Engineering, Yantai University, Yantai, China, 2University of Pennsylvania, Philadelphia, PA, United States, 3Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 4Children’s Hospital of Philadelphia; University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Diffusion tensor imaging (DTI) plays an important role in the diagnosis and prognosis of neonatal hypoxic ischemic encephalopathy (HIE). However, studies on DTI in HIE neonates with normal DWI, T1/T2-weighted images (referred as conventional MRI) are rarely reported. Here, we compared fractional anisotropy (FA), mean diffusivity, radial diffusivity, axial diffusivity among controls, HIE neonates with normal conventional MRI, and HIE neonates with abnormal conventional MRI. The results demonstrate that FA is the most sensitive DTI measurement, and significantly decreased in HIE neonates with normal MRI, suggesting the importance of inclusion of DTI for evaluating HIE in clinical practice and research.
INTRODUCTION
Diffusion tensor imaging (DTI) [1] plays an important role in the diagnosis and prognosis of neonatal hypoxic ischemic encephalopathy (HIE), but the studies on diffusion tensor imaging in HIE neonates with normal DWI, T1/T2-weighted images (referred as conventional MRI) are rarely reported. We hypothesized that the changes of DTI measurements can be found in HIE neonates even with normal conventional MRI by comparing fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), axial diffusivity (AD). DTI data were acquired from 67 term neonates including controls, HIE neonates with normal conventional MRI (HIE/MRI⊖), and HIE neonates with abnormal MRI (HIE/MRI⊕). We aimed to compare those DTI measurements among the 3 groups to evaluate possible alterations in both HIE/MRI⊖ and HIE/MRI⊕ groups.METHODS
Data acquisition: DTI data of 14 controls (male/female 8/6, 16.3±6.5 days), 38 HIE/MRI⊖ neonates (male/female 24/14, 8.8±6.2 days), and 15 HIE/MRI⊕ neonates (male/female, 6.4±4.5 days) were identified for conducting the retrospective case-control study following an IRB approved protocol. Cases were defined according to clinical diagnosis of HIE on medical record and conventional MRI reading. Controls were neonates with normal neurological evaluation, normal conventional MRI, and no clinical signs of HIE. Controls had no seizures, desaturations requiring intervention, acidemia, alterations on the neurological exam during hospitalization, or clinical neurological or cognitive impairment during follow-up. Indications for neuroimaging included baseline anatomical assessment for congenital diaphragmatic hernia, assessment of head/neck masses, and concern for structural alterations in genetic diseases. The DTI images were acquired on a 3T Siemens Magnet on Skyra scanner using the following parameters: TR/TE = 7600/71ms, in-plane field of view (FOV) = 256x256 mm2, in-plane resolution = 2x2 mm2, slice thickness=2mm, number of slices = 42-50, number of gradient directions = 30, b-value = 1000 s/mm2.Image processing and statistical analysis: DTI studio [2] was adopted for DTI data preprocessing including motion correction by AIR registration, as well as the calculation of FA, MD, RD and AD maps. Tract-based spatial statistics (TBSS) [3] was adopted to nonlinearly register those maps to the Penn_CHOP neonate brain atlas [4], followed by projecting the maps onto an alignment-invariant tract representation of skeleton. The FA, MD, RD and AD maps were regressed by age and gender before group comparison to eliminate their effects. Group comparison was then performed voxel by voxel from the skeleton using nonparametric permutation inference (randomize in FSL [5]) among controls, HIE/MRI⊖, and HIE/MRI⊕ groups. P-value of less than 0.05 was considered statistically significant.
RESULTS
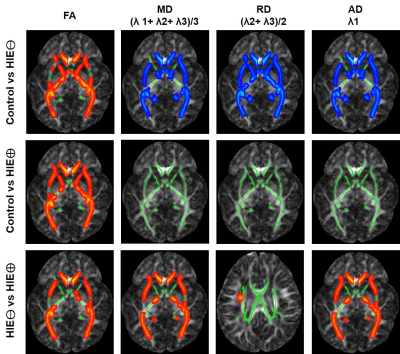
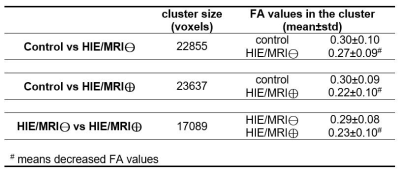
The results demonstrate FA values significantly decreased in HIE/MRI⊖ group, when compared with controls (Figure 1), and further significantly decreased in HIE/MRI⊕ group (1st column in Figure 2). Table 1 gives the cluster size and FA values (mean and standard deviation) of the entire identified skeleton voxels by different group comparison.The MD, RD and AD are significantly altered between controls and HIE/MRI⊖ group, but all the three measurements show no significance between controls and HIE/MRI⊕ group (2nd-4th columns in Figure 2). Besides, both MD and AD are significantly decreased in HIE/MRI⊕ group when comparing with HIE/MRI⊖ group (2nd and 4th columns in Figure 2), while RD demonstrates less significant voxels between HIE/MRI⊖ and HIE/MRI⊕ groups (3rd column in Figure 2).
DISCUSSION and CONCLUSION
Our data demonstrates that FA is the most sensitive DTI measurement comparing with MD, RD, and AD in neonatal HIE detection, and decreased in neonates even from HIE/MRI⊖ group, suggesting the importance of inclusion of DTI for evaluating HIE in clinical practice and research. Whether DTI may serve as a better tool for HIE diagnosis and prognosis, warrants further study in correlation with long-term outcomes.Acknowledgements
This work was supported by National Institutes of Health (KL2TR001879), National Natural Science Foundation of China (61802330), International Postdoctoral Exchange Fellowship Program (20160032), and China Postdoctoral Science Foundation (2015M581203).References
1. Mori, S. and J. Zhang, Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron, 2006. 51(5): p. 527-539.
2. Jiang, H., et al., DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed, 2006. 81(2): p. 106-116.
3. Smith, S.M., et al., Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 2006. 31: p. 1487-1505.
4. Feng, L., et al., Age-specific gray and white matter DTI atlas for human brain at 33, 36 and 39 postmenstrual weeks. NeuroImage, 2019. 185: p. 685-698.
5. Winkler, A.M., et al., Permutation inference for the general linear model. NeuroImage, 2014. 92: p. 381-397.
Figures