4611
Effect of Sedation on Cerebral Metabolic Rate of Oxygen (CMRO2) in Children with Congenital Heart Disease1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2The Children's Hospital of Philadelphia, Philadelphia, PA, United States
Synopsis
CMRO2 was evaluated by MR susceptometry. The hypothesis that CMRO2 is lower in sedated young children with single ventricle heart defects (SVD) was investigated in this study. The results show that (1) lower CMRO2 was found in young subjects after administration of sevoflurane, compared to their unsedated peers, (2) brain volumes in patients with SVD were comparable to those reported in healthy children, (3) cerebral blood flow was smaller than the values reported in healthy subjects. The preliminary data indicate that results from awake and sedated subgroups cannot be pooled, at least not for the study of the neurometabolic consequences.
INTRODUCTION
Children with single ventricle heart defects (SVD) are known to have higher incidence of neurodevelopmental deficits.1 To study the relationship of neurovascular-metabolic effects and increased risk of brain injury in SVD children, cerebral metabolic rate of oxygen (CMRO2) is evaluated by MR susceptometry2 in addition to perfusion and structural metrics in a cohort study. Anesthetics, commonly administered to ensure younger children’s compliance during scanning, have been associated with neurovascular-neurometabolic decoupling, as a result of reduced brain activity.3 Specifically, volatile inhalational anesthetics induce a decrease in CMRO2 and generate a small change in total cerebral blood flow (tCBF) in adults.4 In this ongoing study conducted at 1.5T field strength, we investigated the hypothesis that CMRO2 is lower in sedated patients compared to their unsedated counterparts.METHODS
Fifty subjects with SVD (mean age 9.9 years, height 133.1 cm, weight 32.4 kg) were enrolled. In children indicated for anesthesia (n=36), sevoflurane was administered prior to imaging. A two-slice interleaved OxFlow sequence (Fig. 1) was employed to simultaneously quantify cerebral venous oxygen saturation (SvO2) in the superior sagittal sinus along with whole-brain cerebral blood flow rate (CBF) by phase-contrast velocimetry in the internal carotid and vertebral arteries. SaO2 was obtained by pulse oximeter and CMRO2 was derived from the three measured parameters using Fick’s principle.Imaging was performed at 1.5T (Siemens Avantofit) using a 20-channel head coil. Imaging parameters for OxFlow protocol were: 1x1x5 mm3 voxel size, flip angle of 25°, TR/TE1/ΔTE=35/5/7.04 ms in the head level for SvO2, and 0.85x0.85x5 mm3 voxel size, flip angle of 20°, TR/TE=35/7.22 ms, VENC=60 cm/sec in the neck level for CBF. MPRAGE images used for calculation of brain volume were segmented into grey and white matter using SPM12. CBF was then normalized to 100g of brain tissue, termed as tCBF.
Given that CBF and brain volume increase with age in children,5 possible associations between CBF and brain volumes versus age were examined as potential confounders for CMRO2 estimation. Group analysis was performed using Wilcoxon rank-sum test to determine significance of differences between unsedated and sedated groups. Pearson correlation coefficient was used for a relationship between parameters. A P-value < 0.05 was considered as the criterion for statistical significance.
RESULTS
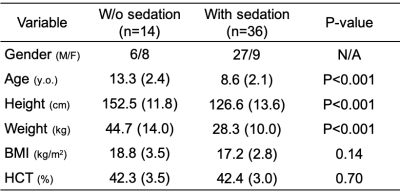
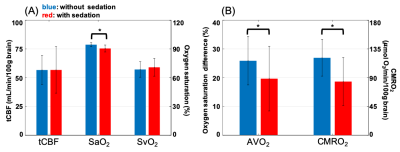
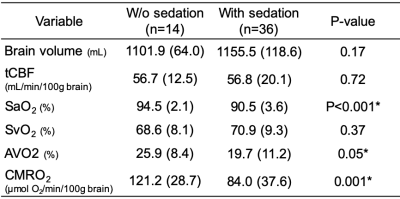
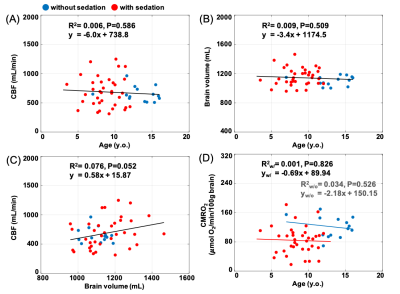
Table 1 lists the demographics of subjects without (n=14) and with sedation (n=36). Figure 2A displays group comparisons in tCBF, SaO2, and SvO2, indicating no significant differences in tCBF and SvO2 between groups. In contrast, awake patients had significantly greater CMRO2 (121.2 versus 84.0 μmol O2/min/100g brain, P=0.001), an effect governed by greater AvO2 (arteriovenous oxygen difference) in the former. The physiological quantities in patients with SVD are summarized in Table 2. These results are in agreement with a study assessing the effects of anesthetics on CMRO2 in adults, which found a 26% reduction of CMRO2 following administration of sevoflurane.3 In addition, brain volume was in good agreement with results of healthy children from a cohort study (this study:1141±108 mL versus reported healthy subjects:1101±210 mL). On the other hand, CBF was 38% lower than reported for healthy subjects (680±228 versus 1102±258 mL/min).5 Figures 3A and 3B indicate that neither CBF nor brain volume were correlated with age (r2=0.006, P=0.59; r2=0.009, P=0.51). A previous study suggests that CBF and brain volume change with age in children. The lack of such a relationship may imply insufficient oxygen transport in children with SVD, which could explain retarded structural and physiological development. Further, CBF was not correlated with brain volume in young patients with SVD (r2=0.076, P=0.052, Fig. 3C). There was no correlation between CMRO2 and age within subgroups (unsedated: r2=0.034, P=0.53, sedated: r2=0.001, P=0.83), confirming that the lower CMRO2 is solely due to the effect of anesthesia (Fig. 3D). Note that CBF, tCBF, brain volume, and age were not correlated within subgroups (data not shown).CONCLUSIONS
The study confirms the hypothesis that CMRO2 in children with SVD is lower in those with relative to those examined without sedation and therefore results from the two subgroups cannot be pooled, at least not for the study of the neurometabolic consequences of the disorder. Even though children in sedated group were younger, age can be excluded as a possible confounding factor. In conclusion, the result of CMRO2 is in well agreement with a study assessing the effects of anesthetics on CMRO2 in adults. However, the results obtained need corroboration given the unbalanced sample sizes between groups.Acknowledgements
This work was supported by the National Institutes of Health (R01 HL090615).References
1. Mahle, W.T., et al., Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome.Pediatrics, 2000. 105(5): p. 1082-9.
2. Jain, V., et al., Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics.J Cereb Blood Flow Metab, 2014. 34(3): p. 380-8.
3. Oshima, T., et al., Effects of sevoflurane on cerebral blood flow and cerebral metabolic rate of oxygen in human beings: a comparison with isoflurane.Eur J Anaesthesiol, 2003. 20(7): p. 543-7.
4. Kitaguchi, K., et al., Effects of sevoflurane on cerebral circulation and metabolism in patients with ischemic cerebrovascular disease.Anesthesiology, 1993. 79(4): p. 704-9.
5. Wu, C., et al., Age-Related Changes of Normal Cerebral and Cardiac Blood Flow in Children and Adults Aged 7 Months to 61 Years.J Am Heart Assoc, 2016. 5(1).
Figures