4604
Longitudinal Assessment of Third Trimester Regional Cerebral Blood Flow in Very Preterm Infants1Division of Diagnostic Imaging and Radiology, Children's National Hospital, Washington, DC, United States, 2Division of Fetal and Transitional Medicine, Children’s National Hospital, Washington, DC, United States, 3Department of Pediatrics, George Washington University, Washington, DC, United States, 4Department of Radiology, George Washington University, Washington, DC, United States, 5Division of Biostatistics and Study Methodology, Children's National Hospital, Washington, DC, United States, 6Division of Neonatology, Children's National Hospital, Washington, DC, United States, 7Division of Neurology, Children's National Hospital, Washington, DC, United States, 8Department of Neurology, George Washington University, Washington, DC, United States
Synopsis
Early newborn age is a crucial phase of brain development, which can be characterized by in-vivo measurement of cerebral blood flow (CBF). In this study we assessed regional CBF in very preterm infants longitudinally during the ex-utero third trimester using arterial spin labeling. CBF in preterm infants significantly increased with postmenstrual age in all regions with the most rapid increase and the highest mean of CBF in the cerebellum. Lower CBF was associated with intraventricular hemorrhage and patent ductus arteriosus. CBF in preterm infants was higher than that of healthy full-term controls at term equivalent age.
INTRODUCTION
Early newborn age is a crucial phase of brain development, which requires adequate supply of oxygen and nutrients to brain tissue delivered by cerebral blood inflow (CBF). Several prior studies have studied CBF in preterm-born infants using arterial spin labeling (ASL), most at term-equivalent age (TEA) or cross-sectionally in the third trimester.1-4 In this study, we assess regional CBF in very preterm infants longitudinally over the third trimester and determine whether CBF is associated with clinical factors.METHODS
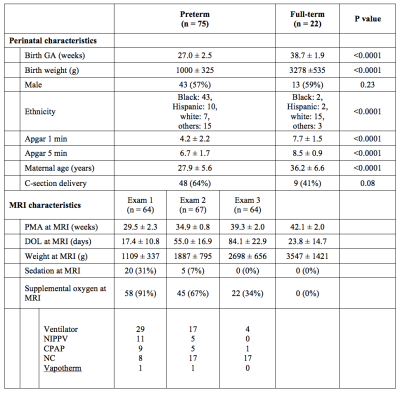
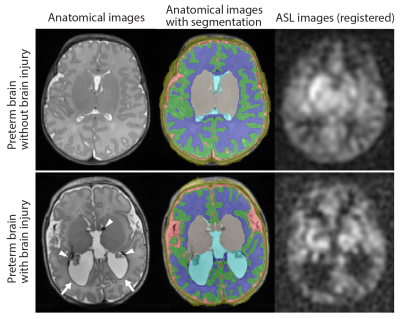
Preterm infants with gestational age at birth ≤ 32 weeks and birth weight ≤ 1500 grams were enrolled in a prospective study that included three longitudinal MRI exams during the ex-utero third trimester period. Healthy full-term control infants were concurrently enrolled for one term MRI exam. The first two MRIs for preterm infants prior to reaching TEA were performed on GE 1.5 T MR450 with a 1-ch incubator coil and all TEA scans (for preterm and term controls) were performed on 3 T MR750 with a standard 8-ch head coil. At each exam, CBF images were acquired using pseudocontinuous ASL and 3D fast-spin-echo stack-of-spirals image readout with imaging parameters of TE = 11 ms, TR = 4.7-4.9 s, FOV = 12-16 cm, in-plane resolution = 2.7-3.5 mm3, slice thickness = 3-4 mm3, number of slices = 22-36, labeling duration = 1450 ms, and postlabeling delay = 2025 ms. Total scan time of ASL was approximately 5 min. For CBF quantification, T1b of each preterm infant was estimated from measured hematocrit value using their relationship found in previous studies: 1/T1b = 1.1 Hct + 0.26 at 1.5 T5 and 1/T1b = 0.5 Hct + 0.37 at 3 T6. In full-term healthy infants, however, T1b was assumed to be 1810 ms for their scans at 3 T.2 Reconstructed CBF images were registered to T2-weighted anatomical images which were automatically segmented to generate the masks of the following regions of interests (ROI): cortical gray matter (CGM), white matter (WM), deep gray matter (DGM), and cerebellum. Mean CBF of these regions as well as global CBF were calculated. TEA scans in preterm infants were graded for parenchymal abnormality (by Kidokoro score)7 and intraventricular hemorrhage (IVH)8. We also examined association between CBF and presence of patent ductus arteriosus (PDA).RESULTS
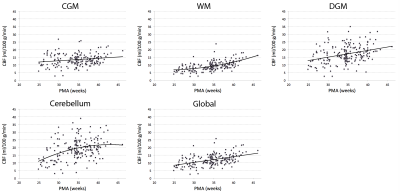
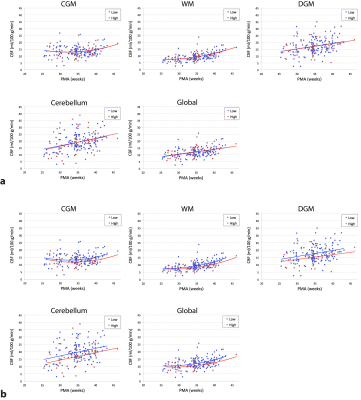
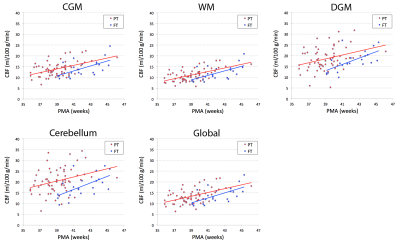
A total of 75 preterm infants and 22 full-term infants were studied, whose baseline characteristics are summarized in Table 1. When controlling for postmenstrual age (PMA), there was no significant difference in global CBF measurement between 1.5 T and 3 T (p = 0.76), suggesting that data analysis with no distinction between the two field strengths is reasonable. The mean CBF in preterm infants was 13.5 ± 3.8, 9.4 ± 3.1, 17.0 ± 5.7, 18.8 ± 6.8, and 11.9 ± 3.6 ml/100 g/min for CGM, WM, DGM, cerebellum and global ROI, respectively (p < 0.0001). As shown in Figure 2, CBF significantly increased with advancing PMA in all regions (p < 0.0001). The cerebellum showed both the highest mean CBF and the most rapid increase in CBF (i.e. highest linear β). CBF was lower in all brain regions (p ≤ 0.05) in infants with higher grade (III or IV) than lower grade (none, I or II) of IVH (Figure 3). However, there were no significant differences in CBF between preterm infants with low (≤ 7) and high (> 7) scores of parenchymal abnormality (p > 0.6). Full-term infants showed significantly lower CBF in all regions compared to preterm infants (p < 0.01) (Figure 4). Infants with conservatively managed PDA showed lower CBF compared to those without PDA in WM (p = 0.03), DGM (p = 0.04), and global ROI (p = 0.05), although there was no significant difference in CBF between preterm infants with ligation treatment and those without PDA.DISCUSSION
We report for the first time longitudinal trajectories in regional CBF in preterm infants during the ex-utero third trimester. Global and regional CBF was found to significantly increase with advancing PMA, perhaps to meet growing metabolic demands during this critical period of brain development. Despite no prior reports of cerebellar CBF in newborns, our findings of the cerebellum are corroborated by previous studies that show the most rapid volume growth during the third trimester in both fetuses and preterm infants.9,10 Association of lower CBF with IVH and PDA may reflect the vulnerability of the preterm brain to hemodynamic instability, and higher CBF in preterm infants (vs full-term) may be attributed to their higher postnatal age.1,4 The predictive value of early CBF for long-term neurodevelopment outcomes in survivors of preterm birth is currently under investigation.Acknowledgements
1U54HD090257References
1. Miranda MJ, Olofsson K, Sidaros K. Noninvasive measurements of regional cerebral perfusion in preterm and term neonates by magnetic resonance arterial spin labeling. Pediatr Res 2006;60:359-363.
2. De Vis JB, Hendrikse J, Groenendaal F, de Vries LS, Kersbergen KJ, Benders MJ, Petersen ET. Impact of neonate haematocrit variability on the longitudinal relaxation time of blood: Implications for arterial spin labelling MRI. Neuroimage Clin 2014;4:517-525.
3. Ouyang M, Liu P, Jeon T, Chalak L, Heyne R, Rollins NK, Licht DJ, Detre JA, Roberts TPL, Lu H, Huang H. Heterogeneous increases of regional cerebral blood flow during preterm brain development: Preliminary assessment with pseudo-continuous arterial spin labeled perfusion MRI. Neuroimage 2017;147:233-242.
4. Bouyssi-Kobar M, Murnick J, Brossard-Racine M, Chang T, Mahdi E, Jacobs M, Limperopoulos C. Altered Cerebral Perfusion in Infants Born Preterm Compared with Infants Born Full Term. J Pediatr 2018;193:54-61.e2.
5. Spees WM, Yablonskiy DA, Oswood MC, Ackerman JJ. Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T(1), T(2), T*(2), and non-Lorentzian signal behavior. Magn Reson Med 2001;45:533-542.
6. Varela M, Hajnal JV, Petersen ET, Golay X, Merchant N, Larkman DJ. A method for rapid in vivo measurement of blood T1. NMR Biomed 2011;24:80-88.
7. Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol. 2013;34:2208-2214.
8. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–534.
9. Andescavage NN, du Plessis A, McCarter R, Serag A, Evangelou I, Vezina G, Robertson R, Limperopoulos C. Complex Trajectories of Brain Development in the Healthy Human Fetus. Cereb Cortex 2017;27:5274-5283.
10. Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics 2005;115:688-695.
Figures