4602
20-min Pediatric Cardiovascular Magnetic Resonance without Contrast or Anesthesia: Initial Evaluation in Kids with Congenital Heart Disease1Department of Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 2Department of Biomedical Engineering, Northwestern University, Evanston, IL, United States, 3Department of Medicine (Cardiovascular Division), Beth Israel Deaconess Medical Center & Harvard Medical School, Boston, MA, United States, 4Division of Pediatric Cardiology, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States
Synopsis
Cardiovascular MRI is an excellent diagnostic tool for children with congenital heart disease (CHD), but the long acquisition times, and need for sedation/anesthesia and the administration of a gadolinium-based contrast results it only accounts for only 2% of pediatric cardiac diagnostic tests. Our 20-min, free-breathing, non-contrast cardiovascular magnetic resonance (CMR) protocol is potentially a game-changer, because its total scan time (20 min) is considerably shorter than a standard protocol (90 min) and does not require anesthesia or gadolinium-based contrast agent.
Introduction
Congenital heart disease (CHD) is the most common type of birth defects, accounting for nearly 1% of live births in the US1,2. Lifetime monitoring, including imaging, is necessary for surviving children with CHD. Cardiovascular MRI is an excellent diagnostic tool for children with CHD. However, cardiovascular MRI accounts for only 2% of pediatric cardiac diagnostic tests due to a variety of factors, including: (a) long scan times (~90 min) and (b) requisite sedation/anesthesia and gadolinium-based contrast agent (GBCA), both of which adds risk and cost and raises potential issues for those children who will require lifetime follow-up3. In this study, we sought to develop and evaluate rapid, free-breathing, non-contrast cardiovascular MRI (real time phase contrast (rt-PC) and real time cine (rt-Cine)) and magnetic resonance angiography (MRA) methods, in order to drastically reduce the scan time to 20 min while producing comparable image quality without requiring GBCA or anesthesia.Methods
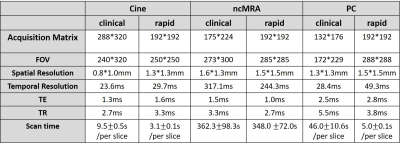
Human Subjects & Pulse Sequence: We enrolled 9 patients (2 females, 7 males, mean age = 9.5 ± 1.6 years) who are scheduled to undergo clinical pediatric CMR (4 with anesthesia; 6 with GBCA; 1 with ferumoxytol; 2 without any contrast agent; all standard clinical doses). Prior to clinical CMR, we conducted our 20-min protocol, which included localizers, real-time cine, real-time phase-contrast, and free-breathing MRA (without preparation pulse). For relevant imaging parameters, please see Table 1.Image reconstruction: The CS reconstruction was performed off-line on a GPU workstation equipped with MATLAB (R2017a, The MathWorks). To accelerate the reconstruction, we applied software coil compression using PCA (8 virtual coils) and used GPU-based NUFFT4. For rt-cine, CS reconstruction was performed using temporal total variation as the sparsifying transform with normalization weight of 0.05 (30 iterations). For rt-PC, CS reconstruction was performed with temporal total variation and temporal principal component analysis as two sparsifying transforms with normalization weight of 0.001 each (30 iterations). For ncMRA, CS reconstruction was performed with total variation along the respiratory motion and slice dimensions with normalized weight of α=0.00075 and β=0.00015, respectively, in combination with normalized fidelity weight of 0.002.
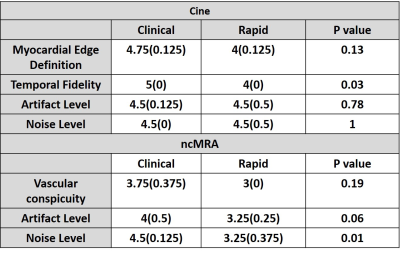
Image quality analysis: Two clinical readers independently graded the images on a 5-point (1: worst, 3: clinically acceptable, 5: best) Likert scale. For rt-cine, 4 categories were: myocardial edge definition, temporal fidelity, noise level, and artifact level. For ncMRA, 3 categories were: vascular conspicuity, noise level, and artifact level. Given the small sample size (N= 9), we assumed non-normal distribution for visual scores and compared two groups using the Wilcoxon signed rank test, where p < 0.05 was considered significant.
Results
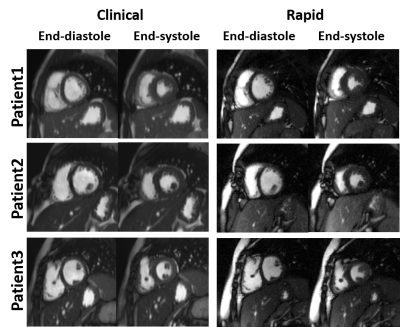
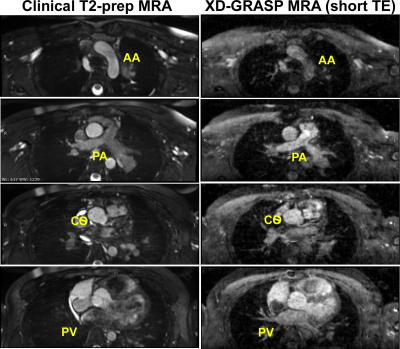
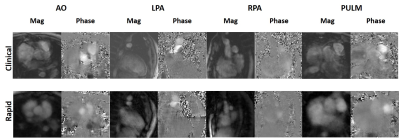
All 9 patients successfully underwent our rapid CMR protocol and the clinical standard CMR protocol. The mean scan time was 98 ± 40 min for clinical CMR and 21 ± 4 min for our rapid CMR, corresponding to 79% reduction in scan time. Figure 1 shows representative cine images of three different patients, illustrating image quality. Figure 2 shows representative ncMRA images of one patient in multiple planes (aortic arch, pulmonary artery, coronary origins, pulmonary vein), illustrating different image quality between clinical and our rapid ncMRA. Figure 3 shows representative phase-contrast images of a patient in 4 different planes, illustrating more blurring with our rapid rt-PC. For rt-Cine, as summarized in Table 2, the median myocardial edge definition, noise scores and artifact scores were not significantly different, whereas the temporal fidelity score was significantly different. Nonetheless, all scores were above the clinically acceptable (3.0) cut point. For ncMRA, as summarized in Table 2, the vascular conspicuity and artifact scores were not significantly different, whereas the noise score was significantly different. Again, all scores were above the clinically acceptable (3.0) cut point.Conclusion
This study describes development and evaluation a 20-min free-breathing, non-contrast CMR protocol for pediatric patients with congenital heart disease. Compared with clinical standard, this protocol reduced the scan time by 79% and eliminate GBCA and anesthesia. Future studies involving more patients and calculation of quantitative metrics (LVEF, aortic dimension, flow) are warranted to further evaluate the clinical utility of 20-min protocol.Acknowledgements
This work is supported by National Institutes of Health (R01HL116895, R01HL138578, R21EB024315, R21AG055954) and the American Heart Association (19IPLOI34760317).References
1. Hoffman JI and Kaplan S. The incidence of congenital heart disease. Journal of the . 2002;39:1890-900. American College of Cardiology
2. Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT and Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. . The Journal of pediatrics 2008;153:807-13.
3. McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE and Eckel LJ. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. 2015;275:772-782.
4. Fessler JA. On NUFFT-based gridding for non-Cartesian MRI. Journal of Magnetic Resonance. 2007;188(2):191-5.
Figures