4600
Altered corticospinal tract microstructure is associated with motor performance in adolescents with congenital heart disease1Child Development Center, University Children's Hospital, Zurich, Switzerland, 2Developmental Pediatrics, Cantonal Hospital Winterthur, Winterthur, Switzerland, 3Cardiology, University Children's Hospital, Zurich, Switzerland, 4Center for MR Research, University Children's Hospital, Zurich, Switzerland
Synopsis
Children with congenital heart diseases (CHD) undergoing open-heart surgery are at an increased risk of motor impairment, but little is known about the neuroanatomical correlates of these deficits. The purpose of the present study was to examine the link between corticospinal tract (CST) microstructure, assessed with diffusion tensor MRI, and motor function in a cohort of adolescents with operated CHD. Compared to age-matched controls, adolescents with CHD showed lower CST anisotropy which correlated with worse fine motor function and poorer movement quality. CHD may therefore lead to alterations in motor tract development, increasing the risk of persistent motor impairments.
Introduction
Congenital heart disease (CHD) represents the most common neonatal malformation, affecting approximately 0.8% of live births.1 With advances in surgical and neonatal care, survival rates have increased and adolescents and adults now outnumber children with CHD.2 However, CHD patients are at an increased risk of neurodevelopmental impairments affecting both the cognitive and motor domains.3 Neuroimaging studies have revealed a link between cognitive deficits and reduced brain volumes4-5 or altered microstructure6 in children or adolescents with CHD, but little is known about the neuroanatomical correlates of persistent motor deficits in this population. The purpose of the present study was to examine the link between corticospinal tract (CST) microstructure and gross and fine motor function in a cohort of adolescents with CHD, in comparison to an age-matched control group.Methods
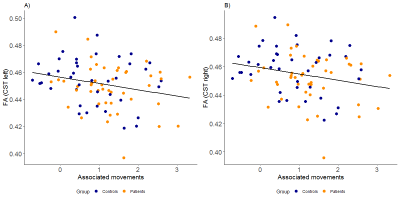
The participant group consisted of 47 adolescents with CHD who underwent full-flow cardiopulmonary bypass surgery before the age of 6 years (mean age at MRI: 13.7 years), and an age matched control group of 44 healthy adolescents (mean age at MRI: 13.9 years). Diffusion tensor imaging (DTI) data were collected using a PGSE sequence with 21 sampling directions, using a GE 3T HD.xt MRI scanner (GE Healthcare, Waukesha, WI, USA). DTI images were skull-stripped and corrected for eddy current effects, and the diffusion tensor was fitted using the tools within the FSL software library. The fractional anisotropy (FA) maps were then normalized into MNI space using the tbss registration tools, and the left and right CSTs from each participant were extracted using the Johns Hopkins University Tractography atlas. Data for the mean FA within each CST were then exported for further statistical analysis.Motor function was evaluated from all CHD patients and 40/44 adolescents in the control group with the Zürich Neuromotor Assessment (ZNA), an extensive battery of motor tests including timed performance tests for gross and fine motor function.7 Briefly, gross motor function was assessed from dynamic and static balance, and fine motor function was assessed with a pegboard test, including an assessment of associated movements.8 IQ was estimated for all participants using the Wechsler Intelligence Scale for Children, 4th edition, (WISC‐IV), and socioeconomic status was assessed using a six point scale based on maternal education and paternal occupation.
Group differences in the CST FA between the patient and control groups were tested with linear regression models correcting for age and sex, and correlations between the FA values and the motor scores in the patient group were assessed with linear regression models, including age and sex as covariates, and Pearson’s R. All statistical analyses were performed with R version 3.5.3.
Results
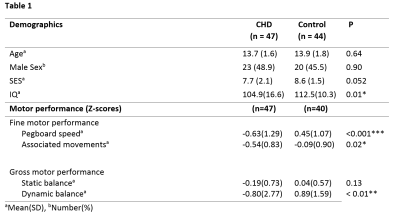
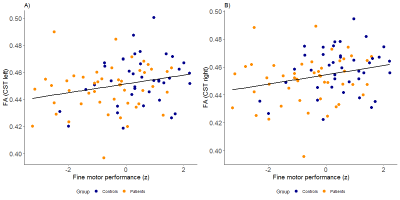
CHD patients demonstrated significantly lower FA in the right CST in comparison to the control group (t(87)=2.105, p=0.04), and a trend towards lower FA in the left CST (t(87) = 1.85, p = 0.07). IQ was within the normal range for both groups, but was lower in the CHD group (t(84)=-2.51, p< 0.01), and a nonsignificant difference in SES was also observed (t(76)=-1.98, p=0.052), with the control group showing a trend towards higher SES. Both groups showed a wide inter-subject variability in gross and fine motor skills, but CHD patients showed significantly lower Z-scores in every domain apart from that of static balance (table 1). FA within the left and right CST correlated inversely with the performance time (left: R=0.24, p=0.04; right: R=0.25, p=0.04) and FA within the left CST correlated with movement quality (i.e. associated movements, R=-0.24, p=0.03) required for performing the pegboard test (figures 1 and 2). However, no significant interactions were present (all p>0.23). Associations between gross motor function tests and CST FA were nonsignificant after including age, sex and group as covariates (all p>0.12).Discussion
Adolescents with CHD show lower CST FA values which were significantly associated with fine motor function and quality, but not with gross motor function. No significant interaction was present, indicating that the relationship between CST FA and fine motor function and quality does not differ between CHD patients and controls.The relationship between CST FA and fine motor function indicates that persistent impairments in fine motor function may arise from atypical development of the primary motor pathway in the presence of a heart defect. The apparent lack of a significant association between CST FA and gross motor function may be due to increased variability, resulting in lower statistical power, or may reflect the importance of other factors such as physical fitness on gross motor function. Alternatively, other pathways outside the CST may also be important for the development of gross motor skills.
Conclusion
Adolescents with congenital heart diseases (CHD) show lower corticospinal tract FA which correlated with fine motor performance and quality. CHD may therefore lead to alterations in motor tract development, increasing the risk of motor impairments.Acknowledgements
Swiss Heart Foundation, Else Kröner-Fresenius FoundationReferences
1. Bernier PL, et al. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13(1):26-34. doi: 10.1053/j.pcsu.2010.02.005.
2. Marelli A, et al. Brain in Congenital Heart Disease Across the Lifespan: The Cumulative Burden of Injury. Circulation. 2016 May 17;133(20):1951-62. doi: 10.1161/CIRCULATIONAHA.115.019881.
3. Schaefer C et al. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. DOI: 10.1111/dmcn.12242
4. Fontes K, et al. Hippocampal alterations and functional correlates in adolescents and young adults with congenital heart disease. Hum Brain Mapp 2019; 40(12): 3548-60.
5. von Rhein M, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain 2014; 137(Pt 1): 268-76.
6. Rollins CK, et al. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr 2014; 165: 936-944.e2
7. Largo RH, et al. Neuromotor development from 5 to 18 years. Part 1: timed performance. Dev Med Child Neurol. 2001 Jul;43(7):436-43.
8. Largo RH, et al. Neuromotor development from 5 to 18 years. Part 2: associated movements. Dev Med Child Neurol. 2001 Jul;43(7):444-53.
Figures