4598
CHemotherapy-Induced cardiotoxicity: LongitudinaL imaging study (CHILL)1Radiology, Children's Hospital Colorado, Aurora, CO, United States, 2Cardiology, Children's Hospital Colorado, Aurora, CO, United States, 3Pediatrics, Children's Hospital Colorado, Aurora, CO, United States, 4Oncology, Children's Hospital Colorado, Aurora, CO, United States, 5Bioengineering, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
Synopsis
In this retrospective study, we explored the potential of cardiac MRI to identify functional imaging biomarkers of cardiotoxicity in pediatric patients exposed to anthracycline chemotherapy. Global and regional myocardial strain was reduced in patients with ejection fraction (EF)>50% indicating that myocardial strain may be an early imaging biomarker of cardiotoxicity in pediatric patients. Additionally, regional differences were found in strain measurements in this population. The results from this study will be used to perform prospective imaging studies to identify early functional imaging biomarkers of cardiotoxicity in pediatric population.
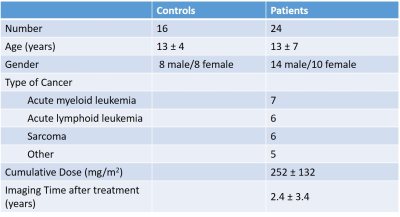
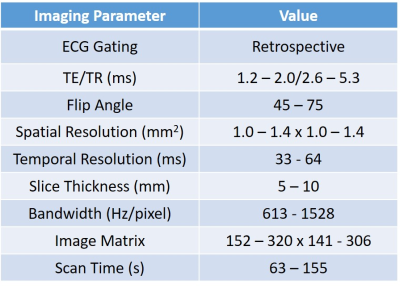
Methods: Sixteen healthy subjects (13±4 years, 8 male and 8 female) and twenty four cancer patients (13±7 years, 14 male and 10 female) who received anthracycline chemotherapy (252±132 mg/m2 doxorubicin equivalent) were retrospectively included via electronic chart review. Table 1 summarizes the patient demographics. Cardiac MRI was performed at 1.5T and 3T (Philips Acheiva/Ingenia and Siemens Avanto). The typical imaging parameters that were used are shown in Table 2. 2D CINE SSFP MRI was performed and tissue tracking software (CIRCLE, Cvi42, Version 5.9) was used to calculate peak radial (Err), peak circumferential (Ecc), peak longitudinal (Ell) strains. Peak systolic radial (SR), circumferential (SC) and longitudinal (SL) strain rates along with peak diastolic radial (DR), circumferential (DC) and longitudinal (DL) strain rates were also calculated. All parameters we computed both on a global level and on a regional level. T2* was computed using CIRCLE (Cvi42 Version 5.9) in the N = 6 patients with EF>50% who had T2* imaging. Cumulative doxorubicin-equivalent anthracycline dose (mg/m2) was recorded for all exposed patients. Significance was computed by T-Test.
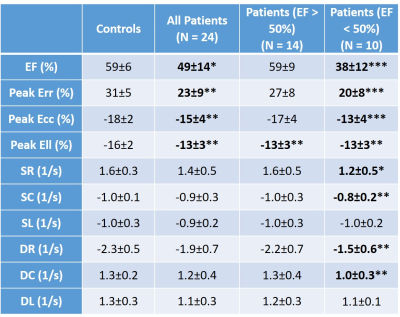
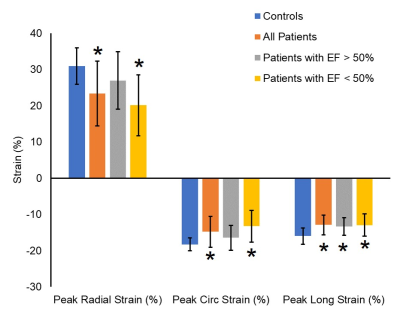
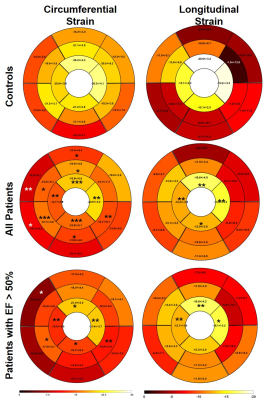
Results: Table 3 summarizes the global strain parameters measured in the control subjects, all patients, patients with EF >50% and patients with EF<50%. LVEF was significantly reduced in the patients (p<0.05 vs. controls). Global peak Err, Ecc and Ell, were significantly reduced in the patients (Table 3 and Figure 1, p<0.05 vs. controls). Additionally, peak longitudinal strain was significantly reduced in patients with EF>50% (Table 3 and Figure 1, p<0.05 vs. controls) indicating that myocardial strain may be a potential early imaging biomarker of cardiotoxicity in pediatric patients. Figure 2 shows regional circumferential and longitudinal strain measurements made in these groups. In patients with EF>50%, apical circumferential and longitudinal strain was reduced (p<0.05 vs. controls) and this spread to both the apical and mid-ventricular segments in the total patient cohort. These results indicate that cardiotoxicity may be heterogeneous in this population thereby requiring careful regional measurements of myocardial strain. In the N=6 patients with EF>50% that had T2* imaging, 33% patients had higher T2* than has been reported in the literature for healthy volunteers. This indicates that T2* may also potentially be an early imaging biomarker of cardiotoxicity, however extensive evaluation is required in a bigger patient cohort to fully validate this.
Conclusion: The results from this study indicate that cardiac MRI can be used to identify early functional imaging biomarkers of cardiotoxicity in a pediatric population. In the future, native T1, T2, T2* and tissue velocity measurements may be included to assess structural changes that may occur earlier than the functional changes examined here.
Acknowledgements
No acknowledgement found.References
1. Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D'Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. American Journal of Cardiology. 2013;112(12):1980-4. Epub 2013/10/01. doi: 10.1016/j.amjcard.2013.08.026. PubMed PMID: 24075281.
2. Pizzino F, Vizzari G, Qamar R, Bomzer C, Carerj S, Zito C, Khandheria BK. Multimodality Imaging in Cardiooncology. Journal of oncology. 2015;2015:263950. Epub 2015/08/25. doi: 10.1155/2015/263950. PubMed PMID: 26300915; PMCID: 4537747.
3. Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the Assessment of Cardiac Injury and Toxicity From Cancer Chemotherapy. A Systematic Review. 2013;6(6):1080-91. doi: 10.1161/circimaging.113.000899.
Figures