4597
Subcortical abnormalities in adolescents with first-episode drug-naïve major depressive disorder1Department of Radiology, the third hospital of Mianyang, Mianyang, China, Mianyang, China, 2Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital, Sichuan University, Chengdu 610041, China, Chengdu, China, 3Department of Psychiatry, the third hospital of Mianyang, Mianyang, China, Mianyang, China
Synopsis
We analysis subcortical nucleus volume in 57 adolescents aged 13-18: 35 with MDD and 22 healthy controls (HC). Compared to HCs, the MDD group showed reduction in volume of the HATA nuclei of the left hippocampus and the subiculum nuclei of the right hippocampus, and increased volume in the LD、MDI、 MDm of the left thalamus and in the MV(Re) of the right thalamus. There were no significant difference in amygdala subregion between the MDD group and the HC group. We propose that these subregions will contribute to the affective and cognitive abnormities in adolescents with MDD.
INTRODUCTION
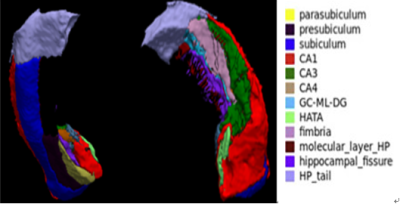
Subcortical structures including hippocampus, thalamus and amygdala play an important role in the mechanism of emotional regulation and thus had long been target for investigation in mental disorder such as major depressive disorder(MDD). All of them are complex structure consisting of functionally distinct nuclei. For example, hippocampal subfields including subfields CA1-4 that make up the cornu ammonis (CA), the dentate gyrus (DG), subiculum, presubiculum, and the fimbria which forms the superior border of the hippocampus. Hence, a detailed subregional analysis of these subcortical nuclei may provide deeper insights into how these abnormalities contribute to symptoms in MDD.Adolescent is a critical time for brain development and the morphometric changes of those structures in MDD for this particular period is worth investigation. In recent year, the new imaging process techniques make possible to study the detailed subregions of those structures. So in current study, we aimed to investigate the subregional anatomical differences of hippocampus, thalamus and amygdala in first-episode drug-naïve MDD adolescents and healthy controls (HC) using 3D T1-weighted imaging and to further explore correlations between age, MDD symptoms and volume of those nuclei.
METHODS
The study was approved by the local ethical committee and written informed consent was obtained from all subjects. A total of 35 first-episode drug-naive adolescents with MDD who met DSM-IV criteria and 22 age, gender and handedness well matched healthy controls were recruited. Illness severity was assessed using the 14-item Hamilton Anxiety Scale (HAMA), the 17-item Hamilton Depression Scale (HAMD) and the Pittsburgh Sleep Quality Index (PSQI). High-resolution T1-weighted images of all the participants were acquired via a Siemens 3.0 T scanner. Volume of subregions of hippocampus, thalamus and amygdala were measured by newest version of FreeSurfer 6.02,3 (http://surfer.nmr.mgh.harvard.edu/).Group comparison of volumes were performed by two sample t-test under SPSS and correlations between clinical parameters and volumes were performed by partial correlation analyses with age, sex, ICV controlled.
RESULTS
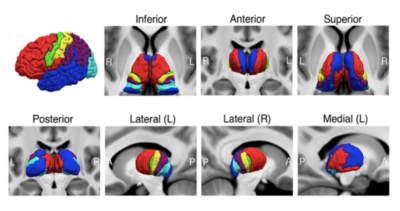
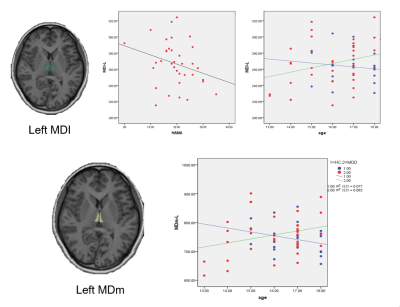
Demographic and clinical characteristics for all subjects are summarized in Table 1. The hippocampal subfields analysis revealed volume reductions in the left HATA nuclei (F =4.200, p =0.045, Table 2) and the right subiculum nuclei (F =4.610, p =0.037, Table 2) in MDD patients relative to HC. However, no significant differences were found in whole hippocampal volume on both hemisphere between the two groups.Compared with HC, adolescents with MDD showed increased volume in the LD, MDI, MDm of the left thalamus and in the MV(Re) of the right thalamus. Significant negative correlation was observed between HAMA scores and volume of MDI in the left thalamus in MDD group(r= -0.468,p=0.007). Significant positive correlation was observed between age and volume of MDI in the left thalamus (r= 0.430,p=0.012), MDm of the left thalamus (r= 0.363,p=0.038) in MDD group. While in HC group, a negative correlation was identified for the same two regions (r= -0.249,p=0.289; r= -0.299,p=0.200).
There were no significant difference in amygdala subregion between the MDD group and the HC group.
DISCUSSION&CONCLUSION
To our best knowledge, this is the first study to characterize the subregional morphometric changes of key subcortical structures in first episode drug-naive adolescents with MDD. Hippocampus is known to be vital for human cognition and emotion processing, and subiculum and HATA nuclei play an important role in gating hippocampus output to other regions of brain4,5,6. Thalamus is a key region in the neural circuit for the exchange of information between cortical and subcortical structures7. The change of volume in some particular regions of those structure may help us understand the neural mechanism of adolescent MDD and deserves further investigation.Acknowledgements
This study was supported by National Nature Science Foundation (Grant NO. 81671669), Science and Technology Project of Sichuan Province (Grant NO. 2017JQ0001).References
1. Shen, X., Reus, L. M., Cox, S. R., Adams, M. J., Liewald, D. C., Bastin, M. E., ... & McIntosh, A. M. (2017). Subcortical volume and white matter integrity abnormalities in major depressive disorder: findings from UK Biobank imaging data. Scientific reports, 7(1), 5547.
2. Sacchet, M. D., Livermore, E. E., Iglesias, J. E., Glover, G. H., & Gotlib, I. H. (2015). Subcortical volumes differentiate major depressive disorder, bipolar disorder, and remitted major depressive disorder. Journal of psychiatric research, 68, 91-98.
3. Han, K. M., Choi, S., Jung, J., Na, K. S., Yoon, H. K., Lee, M. S., & Ham, B. J. (2014). Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. Journal of affective disorders, 155, 42-48.
4. Bora, E., Fornito, A., Pantelis, C. & Yucel, M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. J. Affect. Disord. 138, 9–18 (2012).
5. Voineskos A N, Winterburn J L, Felsky D, et al. Hippocampal (subfield) volume and shape in relation to cognitive performance across the adult lifespan[J]. Human brain mapping, 2015, 36(8): 3020-3037.
6. Zammit A R, Ezzati A, Zimmerman M E, et al. Roles of hippocampal subfields in verbal and visual episodic memory[J]. Behavioural brain research, 2017, 317: 157-162.
7. Nugent, A. C., Davis, R. M., Zarate, C. A. & Drevets, W. C. Reduced thalamic volumes in major depressive disorder. Psychiatry Res. - Neuroimaging 213, 179–185 (2013).
Figures