4595
White Matter Left-lateralization from Birth through Childhood Revealed by Tract-based Spatial Statistics with Dynamic Age-specific Templates1Department of Radiology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi'an, China
Synopsis
Human brain exhibits structural and functional asymmetries. How the left-lateralization in white matter changes with age from newborns through childhood is not fully investigated. In this work, development of the left-lateralization from birth to 13 years is revealed by using tract-based spatial statistics with dynamic age-specific brain templates. Structural left-lateralization with larger fractional anisotropy in motor-related and language-related white matter is already present in newborns. The involvement of corticospinal tract, superior longitudinal fasciculus and uncinate fasciculus into regions with left-lateralization appears at different age. This may be associated with the development of fine motor and language during the early childhood.
INTRODUCTION
Though left and right hemispheres share similar anatomy, the human brain demonstrates structural and functional asymmetries [1, 2]. Structural left-lateralization may be the basis for specific functions, such as the language lateralization and the right handedness [3, 4]. Characterization of the left-lateralization and its development are essential for understanding how the asymmetry arises and abnormal patterns of brain asymmetries in dyslexia, autism, etc [4, 5]. However, how the left-lateralization in white matter microstructure changes with age from newborns through childhood is not fully investigated. In this work, fractional anisotropy (FA) derived from diffusion kurtosis imaging (DKI) is used to characterize the white matter microstructural integrity. Development of the left-lateralization in children with age from 0 (newborns) to 13 years is revealed by using tract-based spatial statistics (TBSS) with dynamic age-specific brain templates.METHODS
This study is approved by the local institutional review board. Informed written consents were obtained from parents of children.Term children (gestational age ≥ 37 weeks) with age from 0 to 13 years were included in this work. Children with history of asphyxia, hypoxic ischemic encephalopathy, central nervous system infection, epilepsy, developmental delay and/or other diseases that may affect brain development were excluded. DKI was performed on a 3T MRI scanner (Signa HDxt; GE Healthcare; Milwaukee, Wisconsin, USA) with an 8-channel head coil. The single shot echo planar imaging sequence for DKI was performed by using the following parameters: b values=0, 500, 1000, 2000, 2500 s/mm2; 18 gradient directions; Repetition time/Echo time =11000/91.7 ms; voxel size= 1.41 × 1.41 × 4 mm3.
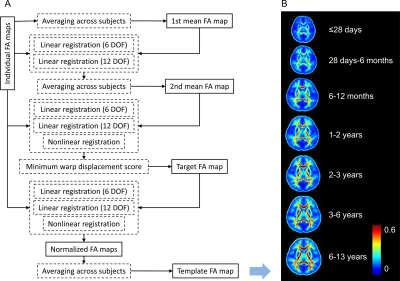
DKI post-processing was performed by using an optimized workflow [6], including the eddy current correction, the artifact rejection, the tensor estimation, and the computation of the derived metrics. To normalize the FA images, age-specific brain templates were created for each age group by using a group-wise template creation method [7] (as shown in Figure 1). In this work, the enrolled children were divided into 7 groups: ≤28 days, 28 days-6 months, 6-12 months, 1-2 years, 2-3 years, 3-6 years, 6-13 years. For each age group, comparisons between left and right hemispheres were performed by using TBSS [8]. To test skeleton voxels on the left hemispheres versus those on the right, symmetric FA skeleton maps were created for each group. All the individual FA images were projected onto the corresponding symmetric FA skeleton. Then all the images were left-right flipped to generate the flipped images. Comparison between left and right hemispheres can be performed by subtracting the flipped images from the original FA images. Tests in TBSS are considered significant at P<0.05 after the family-wise error rate correction and the threshold-free cluster enhancement.
RESULTS
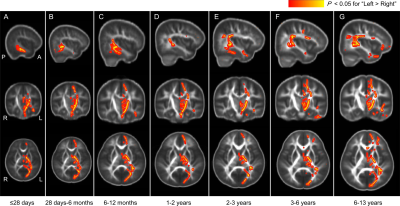
This study enrolls 51 children with age of ≤28 days (35 males), 37 children with age of 28 days-6 months (25 males), 32 children with age of 6-12 months (21 males), 43 children with age of 1-2 years (29 males), 29 children with age of 2-3 years (19 males), 48 children with age of 3-6 years (27 males), and 113 children with age of 6-13 years (78 males).Regions with left-lateralization vary across different age groups (as shown in Figure 2). On newborns, left-lateralization with larger FA can be found on the corticospinal tract (CST) in the cerebral peduncle and the posterior limb of the internal capsule segments. Meanwhile, larger FA is observed on the inferior longitudinal fasciculus (ILF) and/or inferior fronto-occipital fasciculus (IFOF). On children older than 28 days, regions with left-lateralization involve the CST in the centrum semiovale segment. On the children older than 6 months, regions with left-lateralization involve more areas of the parieto-temporal superior longitudinal fasciculus (SLF). On children older than 2 years, fronto-parietal SLF and uncinate fasciculus (UF) are involved. Note that larger FA in the left frontal IFOF can also be found on children older than 3 years.
DISCUSSION
In the researches on correlation between structural and functional asymmetry, motor and language related white matter tracts have been focused [1, 3]. Even on the preterm neonates, microstructural asymmetries have been found in the parieto-temporal SLF and the CST at the cerebral peduncle segment [3]. Similar to this finding, brains of the term newborns exhibit left- lateralization in this current work. These support the hypothesis that prenatal but non-genetic developmental factors determine the structural and functional laterality [9]. Furthermore, results here also demonstrate that the left-lateralization varies with age. Involvement of the centrum semiovale segment of CST in the left-lateralization may be related to development of the fine motor ability in the early childhood. From 2 years on, more areas in the left fronto-parietal SLF and UF demonstrate larger FA than the right hemisphere. This suggests that the age of 2 years is an important milestone age for children to learn language [10].CONCLUDION
Results in this study suggest that structural left-lateralization in motor-related and language-related white matter is already present in newborns. The spatial distribution of white matter tracts with left-lateralization varies with age during the early childhood.Acknowledgements
This study was supported by the National Natural Science Foundation of China (81901823, 81771810, and 81971581), National Key Research and Development Program of China (2016YFC0100300), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education of China (NCET-11-0438), the Project Funded by China Postdoctoral Science Foundation (2019M653659), and the Natural Science Basic Research Plan in Shaanxi Province of China (2019JQ-198).References
[1] Boukadi M, Marcotte K, Bedetti C, Houde JC, Desautels A, Deslauriers-Gauthier S, et al. Test-retest reliability of diffusion measures extracted along white matter language fiber bundles using HARDI-based tractography. Frontiers in Neuroscience 2019;12:1055.
[2] Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science 2002;298(5600):2013-2015.
[3] Liu Y, Baleriaux D, Kavec M, Metens T, Absil J, Denolin V, et al. Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: a diffusion tensor imaging and probabilistic tractography study. NeuroImage 2010;51(2):783-788.
[4] Lee CY, Tabesh A, Nesland T, Jensen JH, Helpern JA, Spampinato MV, et al. Human brain asymmetry in microstructural connectivity demonstrated by diffusional kurtosis imaging. Brain Research 2014;1588:73-80.
[5] Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cerebral Cortex 2009;19(2):414-423.
[6] Li X, Yang J, Gao J, Luo X, Zhou Z, Hu Y, et al. A robust post-processing workflow for datasets with motion artifacts in diffusion kurtosis imaging. PLoS One 2014;9(4):e94592.
[7] Li X, Gao J, Wang M, Wan M, Yang J. Rapid and reliable tract-based spatial statistics pipeline for diffusion tensor imaging in the neonatal brain: applications to the white matter development and lesions. Magnetic Resonance Imaging 2016;34(9):1314-1321.
[8] Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 2006;31(4):1487-1505.
[9] Steinmetz H. Structure, functional and cerebral asymmetry: in vivo morphometry of the planum temporale. Neuroscience and Biobehavioral Reviews 1996;20(4):587-591.
[10] Ferguson B, Graf E, Waxman SR. When veps cry: Two-year-olds efficiently learn novel words from linguistic contexts alone. Language Learning and Development 2018;14(1):1-12.
Figures