4592
Investigation of Longitudinal Nonhuman Primate White Matter Maturation using Quantitative R1 Relaxometry1Pediatrics, University of Wisconsin–Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin–Madison, Madison, WI, United States, 3Waisman Center, University of Wisconsin–Madison, Madison, WI, United States, 4Psychiatry, University of Wisconsin–Madison, Madison, WI, United States
Synopsis
Quantitative relaxometry affords a unique opportunity to map dynamic patterns of white matter development. Nonhuman primates offer a valuable model for investigating neurodevelopment, however, NHP studies utilizing relaxometry based approaches have been limited. Here, we utilize R1 relaxometry to map maturation in 35 rhesus macaques scanned across 5 timepoints during the first year of life. R1 is observed to rapidly increase during this period, with average and individual patterns exhibiting a logarithmic-like trajectory. Results highlight the onset of myelin during this period of development and demonstrate relaxometry to be a promising approach for characterizing patterns of early brain development.
Introduction
The development of white matter (WM) microstructure is a cornerstone of brain development. Formed by bundles of myelinated axons, this neural architecture is fundamental to the establishment of brain connectivity and helps facilitate higher order cognition and behavior1,2. Disruptions to the maturation of the WM microstructure can have significant consequences on development, with aberrant maturation leading to alterations of brain communication and connectivity1,2. Indeed, such microstructural alterations are hypothesized to be involved with a variety of neurodevelopmental disorders and neuropsychiatric conditions, including autism spectrum disorders3,4, schizophrenia5, and anxiety and depression6. Still, there remain significant gaps in knowledge regarding how WM microstructure develops during the earliest periods of life, though such information is central to understanding processes of both normative and atypical neurodevelopment.Nonhuman primates (NHP), in particular the rhesus macaque, provide a valuable model for investigating the dynamics of postnatal brain development. Rhesus monkeys share similar social behaviors, physiology, and brain structure to that of humans, as well as display behavioral alterations that are similar to those associated with human psychopathology7,8. Importantly, rhesus monkeys have an accelerated timeline of brain development compared to humans, allowing for longitudinal neuroimaging studies to be carried out over a shorter period of time. However, brain imaging studies of early postnatal development (less than 1 year) in the rhesus macaque have been limited, while even fewer studies have used quantitative R1 relaxometry to examine NHP WM development during this early phase of life.
In this work, we utilized a novel, high resolution MPnRAGE sequence9 to map developmental changes of R1 across the first year of life in the rhesus macaque. Underlying white mater regions were identified using an available NHP atlas10, and longitudinal developmental trajectories of these WM regions were constructed to investigate spatio-temporal patterns of R1 maturation. We hypothesized that R1 would increase over the first year, corresponding to the rapid development of myelin that is known to take place over this period of development.
Materials and Methods
MRI Acquisition: Thirty-five rhesus monkeys (24 females, 11 males) underwent 3T MRI across 5 time points (approximately 3, 7, 14, 25, and 52 weeks) using the novel and high-resolution MPnRAGE sequence9, which generates multiple inversion recovery contrasts that can be used to derive quantitative R1 maps. One animal had 4 timepoints of data collected due to an error with the MRI scanner. Inversion recovery images were reconstructed at an isotropic resolution of 0.47x0.47x0.47 mm3 and R1 maps were subsequently derived. Analysis: To normalize R1 maps, individual structural T1-weighted images from each animal were nonlinearly aligned and these “subject-specific” templates were used to create a population specific template. A final alignment to a template created from a larger cohort of animals11 was additionally performed. Estimated transformations were subsequently applied to the R1 maps in a single interpolation step. WM regions of interest10, defined in the overall template space, were then used to extract mean R1 from each animal across all timepoints. Age-related trajectories of R1 were examined from these WM regions using R (version 3.6.1).Results
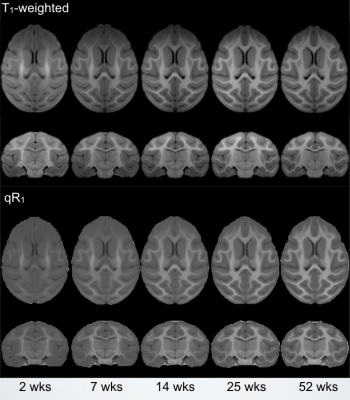
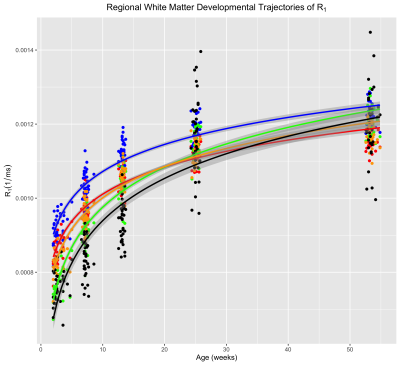
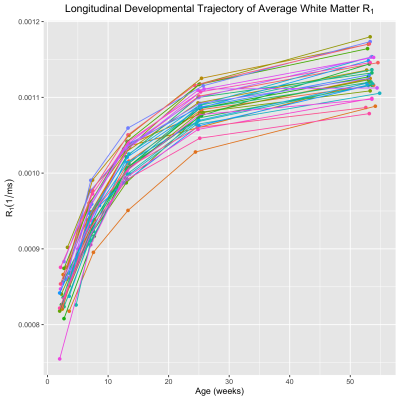
Corresponding axial and coronal slices of the mean structural T1-weighted and R1 maps from the five timepoints are shown in Fig. 1. These data qualitatively illustrate the progressive development of the WM throughout the rhesus brain, indicating a neurodevelopmental trajectory that follows a central-to-peripheral, posterior-to-anterior gradient. Regional developmental trajectories reveal that R1 follows a monotonically increasing shape across the first year of life, appearing to be logarithmic in nature (Fig. 2). While this general logarithmic pattern is consistent between WM regions, there is variability in the extent and rate of change of R1 across different brain regions (Fig. 3). Longitudinal trajectories also indicate that R1 is relatively stable across time, such that animals that have high R1 early in life tend to have higher R1 at later stages and vice versa (Fig. 4).Discussion
The observed changes in R1 reflect the progressive changes of the brain microstructure that occur with development. Developmental changes of R1 throughout this period are likely driven by the elaboration of myelin12, which most rapidly develops during earliest stages of development in both NHP and humans. However, R1 is additionally sensitive to changes in water content, iron accumulation, and macromolecule distributions in tissue13, which may also undergo significant changes throughout early development. The observed regional variation of R1 trajectories is consistent with a limited number of studies that have investigated similar relaxometry based measures during early human brain development14, with trajectories following a center-out, posterior-to-anterior developmental gradient.Conclusion
In this work, we have used quantitative R1 relaxometry to characterize longitudinal WM maturation in the rhesus macaque over the first year of postnatal life for the first time. We have shown that these WM trajectories exhibit distinct nonlinear, logarithmic change over this period, however, future work will systematically investigate mixed-effects growth models that best characterize observed developmental changes. Future work will also investigate relationships between longitudinal R1 measurements and behavioral phenotypes, such as anxious temperament, which were additionally collected. The presented work provides an important step for understanding patterns of early brain microstructural development in the NHP, which holds significant potential for providing new insights into early human neurodevelopment.Acknowledgements
This work was supported by grants P50 MH100031 (Center Director: Richard Davidson, Project PI: Kalin), R00 MH110596 (Dean) from the National Institute of Mental Health, National Institutes of Health. Infrastructure support was also provided, in part, by grant U54 HD090256 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (to the Waisman Center).References
1. Fields, R.D., 2008. White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31, 361-370.
2. Fields, R.D., 2008. White matter matters. Sci Am 298, 42-49.
3. Travers, B.G., Adluru, N., Ennis, C., Tromp do, P.M., Destiche, D., Doran, S., Bigler, E.D., Lange, N., Lainhart, J.E., Alexander, A.L., 2012. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res 5, 289-313.
4. Ameis, S.H., Catani, M., 2015. Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex 62, 158-181.
5. Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93(1):13-24.
6. Sacchet, M.D., Gotlib, I.H., 2017. Myelination of the brain in Major Depressive Disorder: An in vivo quantitative magnetic resonance imaging study. Scientific Reports 7, 2200.
7. Kalin, N.H., Shelton, S.E., 2003. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci 1008, 189-200.
8. Davidson, R.J., Fox, A., Kalin, N.H., 2007. Neural Bases of Emotion Regulation in Nonhuman Primates and Humans. Handbook of emotion regulation. The Guilford Press, New York, NY, US, pp. 47-68.
9. Kecskemeti, S., Samsonov, A., Hurley, S.A., Dean, D.C., Field, A., Alexander, A.L., 2016. MPnRAGE: A technique to simultaneously acquire hundreds of differently contrasted MPRAGE images with applications to quantitative T1 mapping. Magn Reson Med 75, 1040-1053.
10. Zakszewski, E., Adluru, N., Tromp do, P.M., Kalin, N., Alexander, A.L., 2014. A diffusion-tensor-based white matter atlas for rhesus macaques. PLoS One 9, e107398.
11. Fox, A.S., Oler, J.A., Shackman, A.J., Shelton, S.E., Raveendran, M., McKay, D.R., Converse, A.K., Alexander, A., Davidson, R.J., Blangero, J., Rogers, J., Kalin, N.H., 2015. Intergenerational neural mediators of early-life anxious temperament. Proc Natl Acad Sci U S A 112, 9118-9122.
12. Heath, F., Hurley, S.A., Johansen-Berg, H., Sampaio-Baptista, C., 2018. Advances in noninvasive myelin imaging. Dev Neurobiol 78, 136-151.
13. Deoni, S.C., 2010. Quantitative relaxometry of the brain. Top Magn Reson Imaging 21, 101-113.
14. Lebel, C., Deoni, S., 2018. The development of brain white matter microstructure. Neuroimage 182, 207-218.
Figures