4590
Altered functional connectivity of the primary auditory with cognitive networks in inattentive children and adolescents with hearing loss1Radiology, Beijing Tongren Hospital,Capital Medical University, Beijing, China, 2Center for MRI Research, Academy for Advanced Interdisciplinary Studies, Beijing City Key Lab for Medical Physics and Engineering, Institute of Heavy Ion Physics, School of Physics, Peking University, Beijing, China, 3Radiology and Cognitive Imaging Research Center, Michigan State University, East Lansing, MI, United States
Synopsis
Early profound deaf children and adolescents have been reported to suffer from cognitive decline and attentional deficit. Twenty-five inattentive children and adolescents with bilateral prelingual profound deafness and 30 age- and gender- matched normal controls were recruited in this study. The resting state functional connectivity differences were analyzed between two groups. The results indicated a direct impact of early auditory deprivation on high cognitive networks. The usage of hearing aid was also found to normalize the connectivity between primary auditory cortex and attention network.
Introduction
Early profound deaf children and adolescents have been reported to suffer from cognitive decline 1-3, attentional deficit 4-5 and following academic difficulties, which suggest that auditory deprivation may lead to cognitive impairments, especially attentional deficits in profound deaf children and adolescents. Neural correlates of this attention deficits have been little documented. The brain network mechanism of this attention impairment in deaf children and adolescents is not clear yet.Purpose
To investigate the difference of the resting state functional connectivity (RSFC) of the primary auditory cortex with the attention control networks, default mode network and other cognitive networks in inattentive children and adolescents with bilateral early profound deafness.Materials and methods
Twenty-five inattentive children and adolescents with bilateral prelingual profound deafness and 30 age- and gender- matched normal controls were scanned at 3.0 T Siemens MAGNETOM Prisma scanner. High-resolution functional images were acquired using the simultaneous multi-slices echo planar imaging (SMS-EPI) sequence and the high-resolution 3D T1-weighted images were acquired using the magnetization-prepared rapid gradient echo (MPRAGE) sequence. Functional connectivity differences of the superior temporal gyrus and transverse temporal gyrus in primary auditory cortex on two groups were analyzed after data preprocess with AFNI6. Pearson correlation analyses were then used to explore the correlations of functional connectivity changes with the years of deafness, sign language usage, hearing aid usage.Results
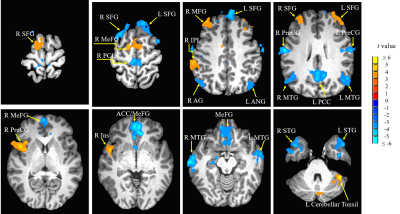
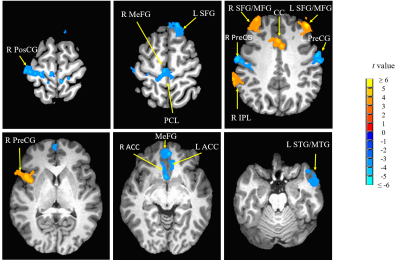
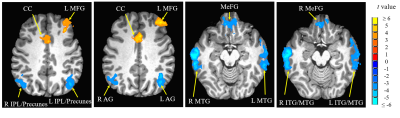
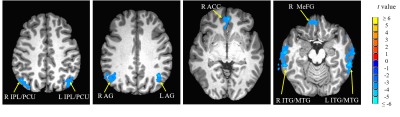
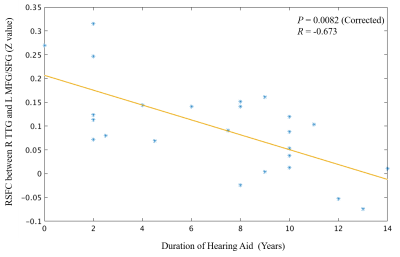
Compared with hearing controls, the deaf subjects showed significantly decreased functional connectivity between seeds and right anterior cingulate cortex and increased functional connectivity between the right superior temporal gyrus and media frontal gyrus, significantly decreased functional connectivity between seeds and middle temporal gyrus and significantly increased functional connectivity with right precentral gyrus. In addition, we also detected both significantly decreased and increased functional connectivity between seeds and left posterior cingulate cortex, bilateral superior frontal gyrus and inferior temporal gyrus as well as left cerebellar tonsil (Fig 1-4). Our results revealed alterations, mainly decrease in resting-state functional connectivity between the primary auditory cortex and the attention control networks, language comprehension networks, default-mode networks and sensorimotor networks in these deaf subjects. The usage of hearing aid was also found to normalize the connectivity between primary auditory cortex and attention network in these deaf subjects (Fig 5).Discussion
We found both significantly decreased and increased RSFC between the primary auditory cortex and the nodes of the attention control networks. Our findings are partially consistent with one of the previous rs-fMRI studies by Ding and colleagues7. Their study found increased functional connectivity between bilateral STG and attention network in deaf adults. Different from their study, in addition to increased RSFC, we mainly found significantly decreased RSFC between primary auditory cortex and the nodes (ACC and MeFG) of the attentional network. The reason for this difference may be due to difference in the research populations. This study focused on deaf children and adolescents while Ding et al. studied early deaf adults. Both impairment and compensation likely happen during the early years of hearing deprivation. The enhanced functional connectivity between the deprived auditory cortex and the attentional network reported in early deaf adults mainly reflected adaptation and compensatory remodeling following the long-term auditory deprivation. The decreased RSFC between primary auditory and attention control networks may contribute to the fundamental reasons of classroom mis-behavior, impaired speech and language acquisition and other cognitive issues in children and adolescents with profound deafness.We also found significantly decreased RSFC between the middle temporal gyrus (MTG) and bilateral STG and TTG due to deafness. The MTG is one of the key nodes in the language comprehension and self-perceptual network8. In daily life, deaf individuals rely on visual information for communication, such as sign language and lip reading, since auditory based language comprehension is impaired. This impairment would severely derail the language development in children and adolescents with prelingual and profound deafness.
Interestingly, in this study, the right precentral gyrus (PreCG) showed increased RSFC with both the right and left STGs. This result is consistent with previous studies7,9. Hand and figure movements are critical to the sign language processing. The increased RSFC between the auditory cortex and the right precentral gyrus could be related to the sign language usage in deaf individuals.
Finally, we found significant negative correlation between the duration of the hearing usage and the RSFC between the right TTG and the left MFG. Left MFG is a node of the attention network. This suggests that the compensatory involvement of attention network was reduced over time with the usage of hearing aid. In other words, the hearing function becomes more normal after the usage of hearing aid. This implies that the hearing aid should be used as early as possible as an intervention tool.
Conclusion
Our findings indicated a direct impact of early auditory deprivation on high cognitive related networks. The usage of hearing aid was also found to normalize the connectivity between primary auditory cortex and attention network.Acknowledgements
This study was supported by National Natural Science Foundation of China (grant number: 81501449). We thank all subjects for their time and effort. We thank Lihua Tai, Xiaocui Wang and Xingxing Fu for their assistance in subject recruitment and hearing testing. We thank Ms. Chunfang Yan and Dr. Xiaoxia Qu in MRI scanning support.References
1. Cupples L, Ching TYC, Leigh G, et al. Language development in deaf or hard-of-hearing children with additional disabilities: typematters! J Intellect Disabil Res. 2018;62(6):532-543.
2. Daza MT, Phillips-Silver J, Ruiz-CuadraMdel M, et al. Language skills and nonverbal cognitive processes associated with reading comprehension in deaf children. Res Dev Disabil. 2014;35(12):3526-33.
3. Hall ML, Eigsti IM, Bortfeld H, et al. Executive Function in Deaf Children: Auditory Access and Language Access. J Speech Lang Hear Res. 2018;61(8):1970-1988.
4. Conway CM, Pisoni DB, Kronenberger WG. The importance of sound for cognitive sequencing abilities: The auditory scaffolding hypothesis. Curr Dir Psychol Sci.2009;18: 275-279.
5. Mitchell TV, Quittner AL. Multimethod study of attention and behavior problems in hearing-impaired children. J Clin Child Psychol. 1996; 25: 83–96.
6. Cox, RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-73.
7. Ding H, Ming D, Wan B, et al. Enhanced spontaneous functional connectivity of the superior temporal gyrus in early deafness. Sci Rep. 2016; 6:23-39.
8. Bonilha L, Hillis AE, Hickok G, et al. Temporal lobe networks supporting the comprehension of spoken words. Brain. 2017; 140: 2370-2380.
9. Li Y, Booth JR, Peng D, et al. Altered intra- and inter-regional synchronization of superior temporal cortex in deaf people. Cereb Cortex. 2013; 23:1988-96.
Figures