4587
Deep learning of electrical stimulation mapping-driven DWI tractography to improve preoperative evaluation of pediatric epilepsy surgery1Pediatrics, Wayne State University School of Medicine, Detroit, MI, United States, 2Translational Imaging Laboratory, Children's Hospital of Michigan, Detroit, MI, United States, 3Translational Neuroscience Program, Wayne State University School of Medicine, Detroit, MI, United States, 4Neurology, Wayne State University School of Medicine, Detroit, MI, United States
Synopsis
To investigate the clinical utility of deep convolutional neural network (DCNN)-tract-classification in the preoperative evaluation of children with focal epilepsy, DCNN-tract-classification deeply learned spatial trajectories of DWI tracts linking electrical stimulation mapping (ESM) findings, and then used to detect eloquent tracts. We found that the DCNN-tract-classification can achieve an excellent accuracy (98%) to detect eloquent areas. Also, the subsequent Kalman filter analysis showed that the preservation of detected areas predicts no postoperative deficits with a high mean accuracy across different functions (92%). Our findings demonstrate that DCNN-tract-classification may offer vital translational information in pediatric epilepsy surgery.
Introduction
Preoperative evaluation of children with focal epilepsy includes localization of the eloquent areas as accurately as possible to identify the resection margin between seizure onset zone and eloquent areas, which assures the postoperative preservation of individual eloquent functions1-3. Electrical stimulation mapping (ESM) via subdural electrodes is considered as the gold standard for delineating the eloquent cortex but does not localize the extent of white matter pathways associated with eloquent function. Furthermore, ESM and functional-MRI are often suboptimal to provide accurate localization in children4-6. The present study tested the hypothesis that the combined application of diffusion-weighted imaging (DWI) tractography and ESM in the framework of deep convolutional neural network (DCNN) learning processes can accurately localize eloquent white matter pathways in pediatric cases.Methods
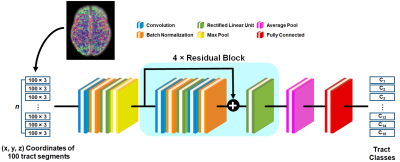
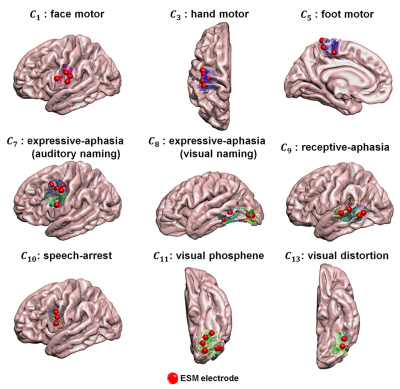
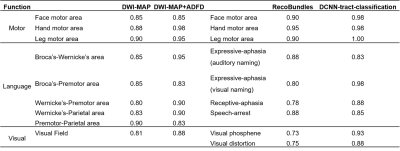
We generated 14 probabilistic maps of eloquent functions in the template space by overlapping ESM-determined eloquent areas across 95 young subjects (12.3±3.4 years) who underwent presurgical ESM via subdural electrodes. These maps were used to define binary seed masks in the subsequent DWI tractography which were acquired using a 3T scanner, generating 14 ESM-driven DWI tract classes in template space, Ci=1-14: C1,2=left,right face motor area-internal capsule, C3,4=left,right hand motor area-internal capsule, C5,6=left,right leg motor area-internal capsule, C7=expressive-aphasia (auditory naming) in left hemisphere, C8=expressive-aphasia (visual naming) in left hemisphere, C9=receptive-aphasia in left hemisphere, C10=speech-arrest in left hemisphere, C11,12=left,right visual-phosphene, and C13,14=left,right visual-distortion. In addition, we defined C15 as an "other" class which includes all tracts from whole-brain tractography not belonging to any of C1-14. All 15 classes were trained together in the DCNN classification in order to discriminate 14 ESM-driven DWI tract classes of interest from any given whole-brain tractography. All tractography analyses were performed using the MRtrix3 package (http://www.mrtrix.org/). Figure 1 shows the architecture of the proposed DCNN, a residual network with 21 layers7. Briefly, each tract was converted to a 100×3 matrix consisting of the 3D coordinates of 100 equidistant segments and input to our DCNN. Both focal loss8 and center loss9 between the prediction and its true membership, Ci, were minimized through iterative optimization via sequential feed-forward/backward propagation. After training, the fully connected layer produced the output probability vector. The class with the highest probability was taken as the final prediction of a given tract. To investigate how effectively the proposed DCNN-tract-classification predicts the likelihood of seizure freedom (benefit) or functional deficit (risk) after surgery, we studied 40 children (age: 9.0±4.9 years) who underwent both pre- and post-operative DWI scans at a 3T scanner. To understand how the retrospective surgical margins suggested by our tract classification model relate to the actual outcomes of surgical resection, we focused on patients with both pre- and post-operative DWI, calculating volumetric postoperative change $$$r_i$$$ of our DWI-generated "eloquent" pathways as (volume of preoperative Ci $$$\cap$$$ volume of resected tissue)/volume of preoperative Ci), and we then determined resection margin $$$d_i$$$ by calculating the minimal Euclidean distance between voxels of Ci and the actual surgical resection boundary. In cases where Ci was resected, $$$d_i$$$ was assumed to be negative and calculated as (-1) × the average Euclidean distance between every voxel inside the resected Ci. To identify the hidden relationship between the preoperatively “unmeasurable” $$$r_i$$$ and measurable $$$d_i$$$, we employed a modified Kalman filter10,11. Finally, we defined a preservation zone of Ci satisfying the Kalman-defined margin $$$d^*_i$$$, balancing postoperative seizure-freedom and functional deficits at $$$d_i(r_i)$$$ satisfying P(deficit|$$$d_i(r_i)$$$)=P(seizure-freedom|$$$d_i(r_i)$$$), where P(deficit|$$$d_i(r_i)$$$) and P(seizure-freedom|$$$d_i(r_i)$$$) represent cumulative probability density functions of seizure freedom and functional deficit at $$$d \leq d_i(r_i)$$$, respectively. Computational experiments using the same data set were carried out to compare the performance of the DCNN tract classification with those of other, previously reported tract classification methods (DWI-MAP12, DWI-MAP+ADFD13, RecoBundles14).Results
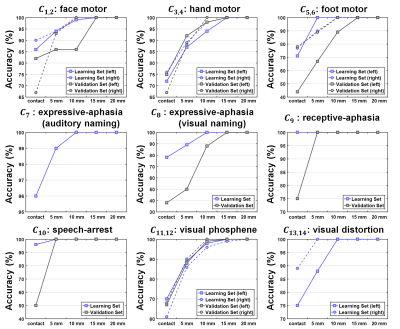
The present study provides two major findings. First, our DCNN-tract-classification model accurately discriminated 14 functionally-important white matter pathways, C1-14, at an average F1 score of 0.993, yielding DCNN-determined cortical terminals that are spatially well-matched to their ground truth data (i.e., eloquent areas determined by ESM) in both the learning set (n=56) and validation set (n=33) where overlap accuracy ranges 86% to 100% within the 1cm spatial resolution of ESM (Figures 2 and 3). Second, the hidden relationships defined by Kalman filter analysis between $$$r_{1-14}$$$ and $$$d_{1-14}$$$ yielded $$$d^*_{1-14}$$$=2.88, 6.12, 1.10, -0.18, -2.72, -1.91, 4.47, -3.53, and -3.66 mm, which ultimately balanced the values of P(deficit|$$$d_i(r_i)$$$) and P(seizure-freedom|$$$d_i(r_i)$$$) for DCNN-tract-classification. The preservation of surgical margin determined by DCNN-tract-classification predicted the avoidance of postoperative deficits at the highest mean prediction accuracy of 92%, compared with DWI-MAP (85%), DWI-MAP+ADFD (90%), and RecoBundles (84%) (Table 1).Discussion
In this study, the clinical utility of DCNN-determined tract pathways was systematically validated by the ESM-based ground truth data. The results indicate that this DCNN-tract-classification method can be a useful tool to non-invasively localize functionally important white matter pathways, which are needed to be preserved in pediatric epilepsy surgery.Conclusion
Our findings demonstrate that postoperative functional deficits are substantially affected by the extent of resected white matter tracts, and that DCNN-tract-classification may offer key translational information by identifying critical pathways whose preservation can optimize functional outcome in pediatric epilepsy surgery.Acknowledgements
This work was supported by grants from the National Institutes of Health(R01 NS089659 to J.J. and R01 NS064033 to E.A.).References
- Lesser RP, Crone NE, Webber WRS. Subdural electrodes. Clin Neurophysiol, 2010; 121:1376-1392.
- Medina LS, Bernal B, Dunoyer C, Cervantes L, Rodriguez M, Pacheco E, Jayakar P, Morrison G, Ragheb J, Altman NR. Seizure disorders: Functional MR imaging for diagnostic evaluation and surgical treatment-prospective study. Radiology, 2005; 236:247-253.
- de Ribaupierre, S, Fohlen M, Bulteau C, Dorfmüller G, Delalande O, Dulac O, Chiron C, Hertz-Pannier L. Presurgical language mapping in children with epilepsy: Clinical usefulness of functional magnetic resonance imaging for the planning of cortical stimulation. Epilepsia, 2012; 53:67-78.
- Haseeb A, Asano E, Juhasz C, Shah A, Sood S, Chugani HT. Young patients with focal seizures may have the primary motor area for the hand in the postcentral gyrus. Epilepsy Res, 2007; 76:131-139.
- Yerys BE, Jankowski KF, Shook D, Rosenberger LR, Barnes KA, Berl MM, Ritzl EK, VanMeter J, Vaidya CJ, Gaillard WD. The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum Brain Mapp, 2009; 30:3426-3435.
- Hamberger MJ, Williams AC, Schevon CA. Extraoperative neurostimulation mapping: results from an international survey of epilepsy surgery programs. Epilepsia, 2014; 55:933-939.
- Xu H, Dong M, Lee MH, O'Hara N, Asano E, Jeong JW. Objective detection of eloquent axonal pathways to minimize postoperative deficits in pediatric epilepsy surgery using diffusion tractography and convolutional neural networks. IEEE Trans. Med. Imaging, 2019; 38:1910-1922.
- Lin TY, Goyal P, Girshick R, He K, Dollar P. Focal loss for dense object detection. In:Proc IEEE Int Conf Vis, 2017; 2980-2988.
- Wen Y, Zhang K, Li Z, Qiao Y. A discriminative feature learning approach for deep face recognition. In: Proc Eur Conf Comput Vis Cham, 2016; 499-515.
- Jeong JW, Asano E, Juhasz C, Chugani HT. Localization of specific language pathways using diffusion-weighted imaging tractography for presurgical planning of children with intractable epilepsy. Epilepsia, 2015; 56:49-57.
- Lee MH, O’Hara N, Motoi H, Luat AF, Juhasz C, Sood S, Asano E, Jeong JW. Novel diffusion tractography methodology using Kalman filter prediction to improve preoperative benefit-risk analysis in pediatric epilepsy surgery. J Neurosurg-Pediatr, 2019; 24:293-305.
- Jeong JW, Asano E, Brown EC, Tiwari VN, Chugani DC, Chugani HT. Automatic detection of primary motor areas using diffusion MRI tractography: Comparison with functional MRI and electrical stimulation mapping. Epilepsia, 2013; 54:1381-1390.
- Lee MH, O'Hara N, Nakai Y, Luat AF, Juhasz C, Sood S, Asano E, Jeong JW. Prediction of postoperative deficits using an improved diffusion-weighted imaging maximum a posteriori probability analysis in pediatric epilepsy surgery. J Neurosurg-Pediatr, 2019; 23:648-659.
- Garyfallidis E, Côté MA, Rheault F, Sidhu J, Hau J, Petit L, Fortin D, Cunanne S, Descoteaux M. Recognition of white matter bundles using local and global streamline-based registration and clustering. Neuroimage, 2018; 170:283-295.
Figures