4586
Functional Connectivity Alterations in Neural Networks Associated with Sustained Attention in Children with Epilepsy1School of Life and Health Sciences & Aston Neuroscience Institute, Aston University, Birmingham, West Midlands, United Kingdom, 2Children’s Epilepsy Surgery Service, Birmingham Children’s and Women’s Hospital NHS Foundation Trust, Birmingham, United Kingdom
Synopsis
Epilepsy is a common neurological disorder diagnosed in children that can be associated with impairments in sustained attention. Attentional deficits are linked to neural dysfunction within or between the default mode network and central executive network. Limited research has examined abnormalities in these networks in children with epilepsy. Using resting-state fMRI we found reduced connectivity within and between both networks in patients (n=18) compared to controls (n=16). The neural alterations found in patients could be potential predictors of attentional deficits, and subsequently aid in identifying children requiring intervention, however further studies are warranted to confirm this.
Introduction
Epilepsy is a commonly diagnosed childhood neurological disorder, affecting 0.5-1% of children below 16 years.1 Importantly, attention is one of the biggest contributors to impaired academic performance in children with epilepsy as compared to other cognitive domains.2, 3 Although epilepsy affect all areas of attention (selective, sustained and divided attention), sustained attention deficit is the most consistently described in epilepsy, and is the most significant predictor of academic performance.4, 5 Attentional deficits are associated with neural dysfunction.6, 7 Recent studies have reported that alterations in the default mode network (DMN) are associated with sustained attention deficits in epilepsy patients.8, 9 Furthermore, DMN connectivity abnormalities extend beyond the DMN itself to other networks including the central executive network (CEN), which may underlie sustained attention deficits in psychiatric disorders.7 This is because both networks need to cooperate to divert attentional resource away from self-referential processes and towards the demands needed by cognitive tasks.10 Moreover, reduced connectivity within the DMN is evident in paediatric epilepsy.11-14 However, the effect of abnormal connectivity between the DMN and CEN underlying sustained attention is not clear, with only one study to date having examined this association in childhood absence epilepsy.15 Thus, this study used resting-state functional magnetic resonance imaging (fMRI) to examine functional connectivity changes within and between the DMN and CEN in a group of children with refractory epilepsy and typically developing children (TDC). It was hypothesised that connectivity within and between these networks would be reduced in patients compared to TDC.Methods
MRI scans from 16 TDC (8-15 years, mean=11.31, 6-males, 10-females) and 18 pre-surgical patients with drug-resistant epilepsy (9-18 years, mean=14.61, 7-males, 11-females), recruited from the community in Birmingham, UK, and Birmingham Children’s Hospital, respectively, were included. T1-weighted structural MR and resting-state fMRI images (TR=3051 ms; TE=30 ms; acquisition time= approximately 5 minutes) were acquired using a 3.0 Tesla Siemens Magnetom Tim Trio scanner at the Aston Brain Centre, Aston University. A paediatric template was generated using the Template-O-Matic toolbox16 and pre-processing (slice-time correction, realignment and scrubbing) was carried out using SPM 1217. Participants with excessive scrubbing (>25%) were excluded.18 To locate regions of interest (ROIs), group ICA was performed on the TDC group. The seed coordinates were determined by peak z-values in the DMN and CEN spatial maps, which were used to create 10mm spherical ROIs, from which averaged time-series were extracted and contrasted using the Network Based Statistics (NBS) toolbox.19 Using t-test, NBS identified brain regions with altered connectivity in patients relative to TDC (threshold, T=2.3, corrected p<0.05).Results
NBS analyses revealed significant resting-state functional connectivity differences between the TDC and patients (corrected p<0.05). Within the DMN, patients showed reduced functional connectivity across several regions relative to TDC (posterior cingulate cortex (PCC) and precuneus; Figure 1). Within the CEN, patients showed reduced functional connectivity between dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PCC) related to TDC (Figure 2). Significant reductions were observed in the connectivity between both networks in patients relative to TDC (parietal cortex, medial prefrontal cortex, PPC and dlPFC; Figure 3).Discussion
The present study investigated differences in resting-state network connectivity within and between the DMN and CEN in a group of children with drug-resistant epilepsy relative to TDC. The reduction found within the DMN replicates similar findings of reduced functional activity in the PCC and precuneus.20 These findings suggest that DMN abnormalities may underlie attentional deficits.21 The reduced functional connectivity within the CEN in patients is also in line with recent findings of reduced connectivity between the fronto and parietal regions in the CEN in a group of adult epilepsy patients relative to controls.22 Given the CEN’s involvement during sustained attention activities, this network dysfunction may also underlie attentional deficits.23 DMN connectivity abnormalities may result in the inability to suppress the DMN during tasks, thus leading to the inappropriate allocation of cognitive resources to perform attentional tasks.6 Likewise, the CEN is associated with top-down attention control, which is vital in sustained attention.24 Therefore, it suggests that disruption within/between these networks can affect the distribution of cognitive resources, where less may be allocated to attention tasks, leading to poorer attention. Previous literature has mainly included adults, and this is one of the first known studies that provides an overview of the neural changes in the networks associated with sustained attention in children with refractory epilepsy. The present findings can aid researchers/clinicians to identify predictors of attentional deficits in children with epilepsy, and early intervention dissemination. Due to the implications of the current findings, future studies should examine the association of these network alterations with behavioural/neuropsychology measures of sustained attention.Conclusion
Paediatric epilepsy patients showed functional connectivity alterations within and between the DMN and CEN as compared to TDC. The findings support other neurological and psychiatric disorders with known attentional deficits, and provide important information on neural alterations that could underlie attentional deficits in children with drug-resistance epilepsy. The neural alterations found are potential predictors of attentional deficits in children with drug-resistance epilepsy, and may aid in identifying children who require attentional interventions, improving their long-term prognosis. Therefore, further studies should examine the association between these alterations to sustained attention performance in epilepsy patients.Acknowledgements
Professor Amanda Wood was supported by a ERC-CoG2015 fellowship (682734-PROBIt).
References
1. Shinnar, S., & Pellock, J. M. (2002). Update on the epidemiology and prognosis of pediatric epilepsy. Journal of Child Neurology, 17(1_suppl), S4-S17.
2. Piccirilli, M., D'Alessandro, P., Sciarma, T., Cantoni, C., Dioguardi, M. S., Giuglietti, M., ... & Tiacci, C. (1994). Attention problems in epilepsy: possible significance of the epileptogenic focus. Epilepsia, 35(5), 1091-1096.
3. Cheng, D., Yan, X., Gao, Z., Xu, K., & Chen, Q. (2017). Attention contributes to arithmetic deficits in new-onset childhood absence epilepsy. Frontiers in psychiatry, 8, 166.
4. Sánchez‐Carpintero, R., & Neville, B. G. (2003). Attentional ability in children with epilepsy. Epilepsia, 44(10), 1340-1349.
5. Williams, J., Phillips, T., Griebel, M. L., Sharp, G. B., Lange, B., Edgar, T., & Simpson, P. (2001). Factors associated with academic achievement in children with controlled epilepsy. Epilepsy & Behavior, 2(3), 217-223.
6. Bonnelle, V., Leech, R., Kinnunen, K. M., Ham, T. E., Beckmann, C. F., De Boissezon, X., ... & Sharp, D. J. (2011). Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. Journal of Neuroscience, 31(38), 13442-13451.
7. Fan, J., Gan, J., Liu, W., Zhong, M., Liao, H., Zhang, H., ... & Zhu, X. (2018). Resting-State Default Mode Network Related Functional Connectivity Is Associated With Sustained Attention Deficits in Schizophrenia and Obsessive-Compulsive Disorder. Frontiers in behavioral neuroscience, 12, 319.
8. Gascoigne, M. B., Smith, M. L., Barton, B., Webster, R., Gill, D., & Lah, S. (2017). Attention deficits in children with epilepsy: preliminary findings. Epilepsy & Behavior, 67, 7-12.
9. Maneshi, M., Moeller, F., Fahoum, F., Gotman, J., & Grova, C. (2012). Resting-state connectivity of the sustained attention network correlates with disease duration in idiopathic generalized epilepsy. PloS one, 7(12), e50359.
10. Kelly, A. C., Uddin, L. Q., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2008). Competition between functional brain networks mediates behavioral variability. Neuroimage, 39(1), 527-537.
11. Oser, N., Hubacher, M., Specht, K., Datta, A. N., Weber, P., & Penner, I. K. (2014). Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS). Epilepsy & Behavior, 33, 12-17.
12. Ibrahim, G. M., Morgan, B. R., Lee, W., Smith, M. L., Donner, E. J., Wang, F., ... & Rutka, J. T. (2014). Impaired development of intrinsic connectivity networks in children with medically intractable localization‐related epilepsy. Human brain mapping, 35(11), 5686-5700.
13. Widjaja, E., Zamyadi, M., Raybaud, C., Snead, O. C., & Smith, M. L. (2013). Abnormal functional network connectivity among resting-state networks in children with frontal lobe epilepsy. American Journal of Neuroradiology, 34(12), 2386-2392.
14. Oyegbile, T. O., VanMeter, J. W., Motamedi, G. K., Bell, W. L., Gaillard, W. D., & Hermann, B. P. (2019). Default mode network deactivation in pediatric temporal lobe epilepsy: Relationship to a working memory task and executive function tests. Epilepsy & Behavior, 94, 124-130.
15. Li, Q., Cao, W., Liao, X., Chen, Z., Yang, T., Gong, Q., ... & Yao, D. (2015). Altered resting state functional network connectivity in children absence epilepsy. Journal of the neurological sciences, 354(1-2), 79-85.
16. Wilke, M., Holland, S. K., Altaye, M., & Gaser, C. (2008). Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage, 41(3), 903-913.
17. Ashburner, J., Barnes, G., Chen, C., Daunizeau, J., Flandin, G., Friston, K., ... & Penny, W. (2014). SPM12 manual. Wellcome Trust Centre for Neuroimaging, London, UK.
18. Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142-2154.
19. Zalesky, A., Fornito, A., & Bullmore, E. T. (2010). Network-based statistic: identifying differences in brain networks. Neuroimage, 53(4), 1197-1207.
20. Laufs, H., Hamandi, K., Salek‐Haddadi, A., Kleinschmidt, A. K., Duncan, J. S., & Lemieux, L. (2007). Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Human brain mapping, 28(10), 1023-1032.
21. Leech, R., & Sharp, D. J. (2013). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(1), 12-32.
22. Alonazi, B. K., Keller, S. S., Fallon, N., Adams, V., Das, K., Marson, A. G., & Sluming, V. (2019). Resting‐state functional brain networks in adults with a new diagnosis of focal epilepsy. Brain and behavior, 9(1), e01168.
23. Sauseng, P., Hoppe, J., Klimesch, W., Gerloff, C., & Hummel, F. C. (2007). Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. European Journal of Neuroscience, 25(2), 587-593.
24. Sylvester, C. M., Corbetta, M., Raichle, M. E., Rodebaugh, T. L., Schlaggar, B. L., Sheline, Y. I., ... & Lenze, E. J. (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in neurosciences, 35(9), 527-535.
Figures
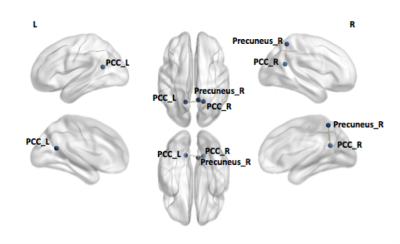
Figure 1. Functional connectivity differences between TDC and epilepsy patients within the DMN
Significant difference was identified using a network based statistics toolbox with a threshold of 2.3 and 5,000 permutations and displayed using Network Brain Viewer toolbox (P< 0.05). Reduction in connectivity between the left posterior cingulate cortex and right precuneus, and the left and right posterior cingulate cortex was found in epilepsy patients compared to TDC.
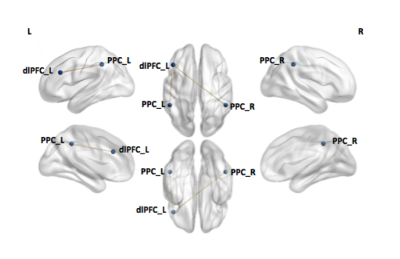
Figure 2. Functional connectivity differences between TDC and epilepsy patients within the CEN
Significant difference was identified using a network based statistics toolbox with a threshold of 2.3 and 5,000 permutations and displayed using Network Brain Viewer toolbox (P< 0.05). Reduction in connectivity between the left dorsolateral prefrontal cortex and the left/right posterior parietal cortex was found in epilepsy patients compared to TDC.
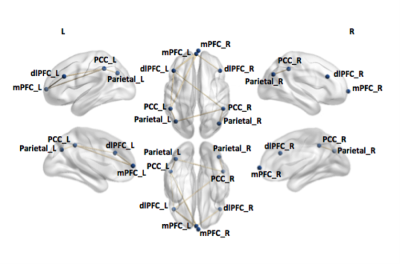
Figure 3. Functional connectivity differences between TDC and epilepsy patients between the DMN and CEN
Using the same threshold, reduction in connectivity between left parietal cortex and left/right posterior parietal cortex (PPC), right parietal cortex and right PPC, left PPC and left/right medial prefrontal cortex (mPFC), right dorsolateral prefrontal cortex (dlPFC) and left mPFC, and left dlPFC and left/right mPFC was found in epilepsy patients compared to TDC.