4584
Altered Cortical Folding Depth in Fetuses with Down Syndrome1Newborn Medicine, Boston Children's Hospital, Boston, MA, United States, 2Radiology, Tufts Medical Center, Boston, MA, United States, 3Obstetrics and Gynecology, Long Island Jewish Medical Center, New Hyde Park, NY, United States, 4Mother Infant Research Institute, Tufts Medical Center, Boston, MA, United States, 5Medical Genetics Branch, National Human Genome Research Institute, Bethesda, MD, United States
Synopsis
Analyzing fetal brains with Down syndrome is an interesting are because genetic factors affect brain development in early fetal stages. In addition to cortical volumetric and areal measures used in our previous studies, cortical folding in the fetal brain would be an important marker of altered neurodevelopment due to Down syndrome. Thus, we calculated sulcal depth to quantify cortical folding and compared sulcal depth between Down syndrome and typically developing fetuses. Down syndrome fetuses showed significantly altered sulcal depth compared to typically developing peers in the regions related to decreased neurogenesis in early fetal life.
Introduction
Down Syndrome (DS) is a genetic disorder caused by trisomy 21. Advanced analysis of brain magnetic resonance (MR) imaging has been used to find brain abnormalities and their relationship with neurodevelopmental deficits in patients with DS1–5.Because genetic factors affect brain development in early fetal life, analyzing fetal brains may provide better understanding of altered brain development caused by DS. Our previous study used global cortical volumetric and areal measures to analyze and compare growth patterns between DS and typically developing (TD) fetuses6. It showed reduction in growth of cortical plate volume and right cortical surface area in DS fetuses compared to TD peers, and demonstrated brain pathology of DS originates in the early fetal life from the second trimester of gestation6. These reduced growth may be associated with altered neurogenesis and neurodevelopmental delay in DS7,8. Affected by neurogenesis9,10, cortical folding is an important marker of brain development11 and is linked to functional localization of the human brain. Therfore, we hypothesized that DS fetuses would show altered cortical folding which is related to decreased neurogenesis and neurodevelopmental deficits. In this study, we measured sulcal depth which is a quantitative measure of cortical folding12, and performed statistical analysis using sulcal depth to find altered cortical folding in DS compared to TD fetuses.
Methods
The study was approved by the local Institutional Review Board at Boston Children’s Hospital (BCH) and Tufts Medical Center (TMC). The subjects of this study consisted of 16 TD (male/female: 6/10, gestational week (GW): 30.5 ± 1.3 [mean ± standard deviation]) and 8 DS fetuses (male/female: 3/5, GW: 30.9 ± 2.3). The brain MR images were acquired on a 3T Skyra scanner (BCH) or Phillips 1.5 T scanner (TMC) using a T2-weighted HASTE (Half-Fourier Acquisition Single-Shot Turbo Spin-Echo) sequence with 1 mm in-plane resolution (FOV = 256mm, time repetition = ~1.5s (BCH) or 12.5s (TMC), time echo = ~120ms (BCH) or 180ms (TMC), and slice thickness = 2~4mm).For MR images, we adopted our fetal MR image processing pipeline6,13,14. Combining multiple 2D slices of fetal brain MR images, we created a motion-corrected 3D volume with 0.75 mm isotropic resolution15. Then, the cortical plate was segmented by a semi-automatic approach based on voxel intensities. The segmented inner volume of the cortical plate was smoothed with 1.5mm full width at half-maximum (FWHM) kernel. Using the smoothed inner volume, the hemispheric triangular surface meshes of the cortical plate were automatically extracted by a function “isosurface” in MATLAB 2016b (MathWorks Inc., Natick, MA). The surface meshes were geometrically smoothed and then aligned to 28 GW template surface using a 2D sphere-to-sphere warping method13,16. The aligned surfaces had vertex-wise correspondence among the individual surfaces. Then, we calculated sulcal depth using adaptive distance transform which searches the shortest path from the convex hull to the cortical surface12. Finally, we smoothed sulcal depth with 5 mm full-width at half-maximum (FWHM) Gaussian smoothing kernel. To assess group difference in sulcal depth, we employed a general linear model at whole-brain average and vertex-wise sulcal depth with controlling for sex and GW. For vertex-wise comparison, multiple comparisons were taken into account using a false discovery rate (FDR) correction at a 0.05 level of significance.
Results
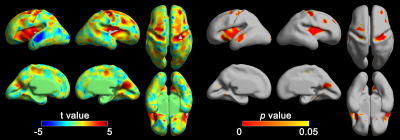
In statistical group analysis, significantly shallower whole-brain average sulcal depth was found in fetuses with DS compared to TD fetuses (t = 2.69, p = 0.007). The vertex-wise sulcal depth in DS was also significantly different compared to TD in several cortical regions including Sylvian fissure, central, precentral, middle frontal, parieto-occipital sulci, and superior temporal sulci which are the early emerging sulci (Figure 1). Significantly shallow sulcal depth in DS was revealed in Sylvian fissure, central, precentral, middle frontal, and parieto-occipital sulci. In contrast, DS fetuses showed significantly deeper sulcal depth in the left superior temporal sulcus than TD fetuses (Figure 1).Discussion
In this study, we globally and regionally compared sulcal depth between TD and DS fetuses. In Sylvian fissure, shallower sulcal depth in DS may be related to prenatal brain abnormalities with developmental delay17. The motor abnormalities in children with DS18,19 may support shallower sulcal depth in DS compared to TD fetuses in bilateral central and precentral sulci. Recently, diffusion tensor imaging studies reported white matter abnormalities such as decreased fractional anisotropy in the fronto-temporal-occipital association tract of DS patients4,5. The white matter abnormalities in DS may support our findings of different sulcal depth in middle frontal, parieto-occipital sulci, and superior temporal sulci. In summary, we found significantly altered sulcal depth in DS compared to TD fetuses, and the findings may be related to neurodevelopmental alterations caused by decreased neurogenesis in early GW.Acknowledgements
This work was supported by National Institutes of Health R21HD094130, K23HD079605, Jerome Lejeune Foundation, and the Susan Saltonstall Foundation.References
1. Guedj, F. & Bianchi, D. W. Noninvasive prenatal testing creates an opportunity for antenatal treatment of Down syndrome: Noninvasive prenatal testing and Down syndrome. Prenat. Diagn. 33, 614–618 (2013).
2. Lee, N. R. et al. Dissociations in Cortical Morphometry in Youth with Down Syndrome: Evidence for Reduced Surface Area but Increased Thickness. Cereb. Cortex 26, 2982–2990 (2016).
3. Carducci, F. et al. Whole-brain voxel-based morphometry study of children and adolescents with Down syndrome. Funct. Neurol. 10 (2013).
4. Gunbey, H. P. et al. Structural brain alterations of Down’s syndrome in early childhood evaluation by DTI and volumetric analyses. Eur. Radiol. 27, 3013–3021 (2017).
5. Baburamani, A. A., Patkee, P. A., Arichi, T. & Rutherford, M. A. New approaches to studying early brain development in Down syndrome. Dev. Med. Child Neurol. 61, 867–879 (2019).
6. Tarui, T. et al. Disorganized Patterns of Sulcal Position in Fetal Brains with Agenesis of Corpus Callosum. Cereb. Cortex 28, 3192–3203 (2018).
7. Guidi, S. et al. Abnormal development of the inferior temporal region in fetuses with Down syndrome. Brain Pathol. 28, 986–998 (2018).
8. Guihard-Costa, A.-M., Khung, S., Delbecque, K., Ménez, F. & Delezoide, A.-L. Biometry of Face and Brain in Fetuses with Trisomy 21. Pediatr. Res. 59, 33–38 (2006).
9. Ronan, L. & Fletcher, P. C. From genes to folds: a review of cortical gyrification theory. Brain Struct. Funct. 220, 2475–2483 (2015).
10. Rakic, P. Genetic Control of Cortical Convolutions. Science 303, 1983–1984 (2004).
11. Garel, C., Chantrel, E., Elmaleh, M., Brisse, H. & Sebag, G. Fetal MRI: normal gestational landmarks for cerebral biometry, gyration and myelination. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 19, 422–425 (2003).
12. Yun, H. J., Im, K., Yang, J.-J., Yoon, U. & Lee, J.-M. Automated Sulcal Depth Measurement on Cortical Surface Reflecting Geometrical Properties of Sulci. PLOS ONE 8, e55977 (2013).
13. Yun, H. J. et al. Automatic labeling of cortical sulci for the human fetal brain based on spatio-temporal information of gyrification. NeuroImage 188, 473–482 (2019).
14. Im, K. et al. Quantitative Folding Pattern Analysis of Early Primary Sulci in Human Fetuses with Brain Abnormalities. Am. J. Neuroradiol. 38, 1449–1455 (2017).
15. Kuklisova-Murgasova, M., Quaghebeur, G., Rutherford, M. A., Hajnal, J. V. & Schnabel, J. A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med. Image Anal. 16, 1550–1564 (2012).
16. Robbins, S., Evans, A. C., Collins, D. L. & Whitesides, S. Tuning and comparing spatial normalization methods. Med. Image Anal. 8, 311–323 (2004).
17. Guibaud, L. et al. Abnormal Sylvian fissure on prenatal cerebral imaging: significance and correlation with neuropathological and postnatal data. Ultrasound Obstet. Gynecol. 32, 50–60 (2008).
18. Vicari, S. Motor Development and Neuropsychological Patterns in Persons with Down Syndrome. Behav. Genet. 36, 355–364 (2006).
19. Palisano, R. J. et al. Gross motor function of children with down syndrome: Creation of motor growth curves. Arch. Phys. Med. Rehabil. 82, 494–500 (2001).
Figures