4581
Whole Brain Cerebral Oxygenation Measurements in Fetuses Using 4D MRI
Jing Liu1, Yan Wang1, Duan Xu1, Patrick McQuillen1, and Shabnam Peyvandi1
1University of California San Francisco, San Francisco, CA, United States
1University of California San Francisco, San Francisco, CA, United States
Synopsis
We developed accelerated 4D MRI and efficient image processing tool for whole fetal brain oxygenation measurement. Fetuses with CHD have lower cerebral oxygenation compared with controls and exhibit an oxygenation increase with maternal hyperoxia testing while controls exhibit no change.
Introduction
Major congenital heart defects (CHD) impair cerebral growth, delay cerebral maturation, and cause neurodevelopmental disorders in children. Studies showed lower cerebral oxygenation in fetuses with major heart defects compared to that of fetuses without heart defects based on T2* measurements in the fetal brain [1]. In this study, we explored T2* mapping for assessing the alterations in the oxygenation properties of the fetal brain. We evaluated the cerebral oxygenation in fetuses with and without heart defects, and investigated their changes before and after maternal hyperoxia (MH). We hypothesized that fetuses with CHD would have lower cerebral oxygenation compared with controls and that they would exhibit a greater response to brief administration of maternal hyperoxia.Methods
A previously developed undersampling strategy allows accelerated dynamic 3D imaging for a variety of applications [2]. In this study, we explored it for achieving highly accelerated dynamic 3D (4D) fetal imaging with multi-echo acquisition for assessing cerebral oxygenation in fetuses. Our technique allows whole brain T2* measurement by a high frame rate (<15s) imaging with motion robustness/compensation in a fairly short scan time (<3mins). This study was approved by the Institutional Review Board (IRB) at the University of California San Francisco (UCSF). Written, informed consent was obtained from all subjects prior to the MR scans. The 4D MRI was performed on 45 subjects (24 CHD patients and 21 controls) on a 3.0T MR scanner (GE Healthcare, Waukesha, WI) equipped with a 32-channel coil. 3D multi-echo SPGR acquisition was applied at base and after maternal hyperoxia. Relevant imaging parameters were: FOV = 32.0×32.0 cm2, slice thickness = 6 mm, image matrix = 160×160×30, TR = 24.0 ms, 8 TEs=1.96~22.0ms with 2.86 ms incensement, flip angle = 20°, and readout bandwidth = ±125kHz. Scan time was ~140s. The continuously acquired data were reconstructed to 3D images at 10 time frames (14s per frame) using a multicoil compressed sensing method (k-t SPARSE-SENSE) [3,4] with an acceleration factor of ~8. For T2* measurement in the fetal brain, 3D images of the time frame with minimal motion artifacts was identified visually for image processing. To achieve accurate and efficient oxygenation measurement in the whole fetal brain, we developed a semi-automatic segmentation tool based on the multi-echo MR acquisition. The main steps of the image processing include: 1) cropping of the fetal brain region (12x12x12 cm3), 2) initial segmentation of the fetal brain by applying the modified k-means clustering on multi-echo data, 3) automatic segmentation of the fetal brain using shape-based Level-Set methods [5,6], 4) registration of the segmented fetal brain at base and after maternal hyperoxia, and 5) T2* fitting based on the averaged signal of the whole brain throughout the 8 echo times.Results and Discussion
Images from total 42 subjects (22 CHD and 20 controls) were included for image processing and data analysis, excluding 3 subjects (2 CHD and 1 control) due to poor image quality. Figure 1 shows the images from a representative case. The conventional 3D acquisition is prone to fetal motion as shown in the first row. The proposed 4D imaging method allows us to catch the time frame with relatively minimal motion. There was obvious fetal movement between the scans in this case. The process to obtain an initial segmentation of fetal brain is shown in Figure 2. Modified k-means clustering allows to initial separate the fetal brain from many other tissues which have different decay curves. Figure 3 shows the fetal images under the processing (brain segmentation and registration). Only the registered, same voxels from scans at base and after maternal hyperoxia were included for T2* calculation. As shown in Table 1, the baseline cerebral tissue oxygenation is lower in CHD vs. control (p<0.05, significant). With Hyperoxia, the T2* values are similar in the two groups. Overall, the change in T2* is 5.3 ms faster in the CHD group compared with the control group (95% CI:-1.4, 12.0, p=0.1), suggesting T2* increases in CHD while it remains overall the same in control. In both groups at baseline, T2* declines with advancing gestational age (GA) at the same rate. T2* declines by 7.1 ms per week increase in GA, (95% CI: -14.8, 0.72, p= 0.07) in control subjects, and by 8.2 ms (95% CI: -14.5, -1.8, p= 0.01) in CHD subjects. With MH, a similar relationship is seen between T2* and GA, though less significant. The change in T2* with MH is not associated with GA for either group.Conclusions
We have implemented accelerated 4D MRI and efficient image processing tool for achieving whole fetal brain oxygenation measurements. Fetuses with CHD have lower baseline cerebral oxygenation compared with controls. In addition, CHD fetuses exhibit an increase in cerebral oxygenation with maternal hyperoxia testing while controls exhibit no change. These findings suggest that decreased oxygen delivery to the brain is one factor that may contribute to delays in brain development in fetuses with CHD.Acknowledgements
No acknowledgement found.References
1. Lauridsen HM, et al. Circ Cardiovasc Imaging, 2017 Nov;10(11):e006459.
2. Liu J, Saloner D, Quant Imaging Med Surg 4: 57-67. 2014.
3. Otazo R, et al. Magn Reson Med. 2010;64:767–776.
4. Feng L, et al. Magn Reson Med. 2013;70:64–74.
5. Wang Y, et al. Med Image Anal. 2017; 40:1-10.
6. Wang Y, et al. Medical physics. 2019; 46:180-189.
Figures
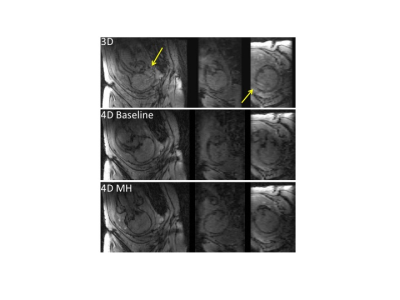
Figure 1. Fetal images acquired with the conventional 3D imaging (first row) and
the proposed 4D MRI (second and third rows for the scans before and after maternal
hyperoxia respectively), displayed at three orthogonal planes (columns). The
conventional 3D acquisition is prone to fetal motion as highlighted with
arrows.
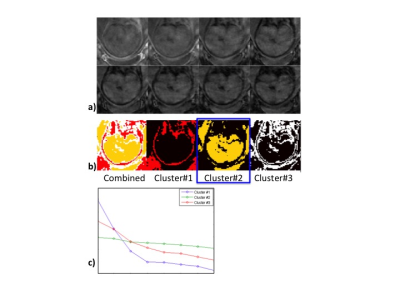
Figure 2. a) fetal brain images at 8 TEs, b) initial segmentation of fetal brain
achieved (cluster#2) by applying modified k-means clustering on the multi-echo
data, which has different T2* decay signal curves (c) from different tissues.
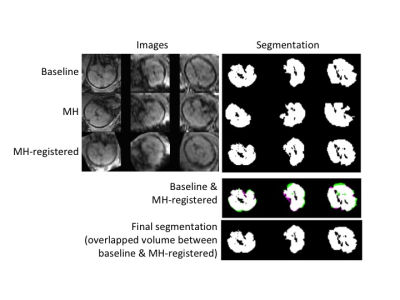
Figure 3. Images from the scans before and after maternal hyperoxia were
segmented and registered to obtain the final segmentation for T2* measurements.
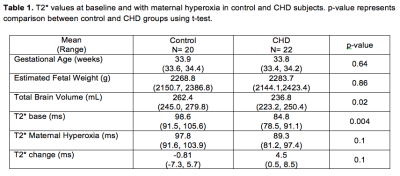
Table 1. T2* values at baseline and with maternal hyperoxia in control and CHD
subjects. p-value represents comparison between control and CHD groups using
t-test.