4580
Neurochemical Correlates of Brain Abnormalities in Prenatal Alcohol Exposure: Interim Analysis
Jeffry R. Alger1,2,3, Joseph O'Neill4, Lisa Kilpatrick4, Katherine L. Narr1, Guldamla Kalender4, Ronald Ly4, Shantanu H. Joshi1, Sandy Loo4, Mary J. O'Connor4, and Jennifer G. Levitt4
1Neurology, University of California, Los Angeles, Los Angeles, CA, United States, 2Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3NeuroSpectroScopics LLC, Sherman Oaks, CA, United States, 4Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, United States
1Neurology, University of California, Los Angeles, Los Angeles, CA, United States, 2Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3NeuroSpectroScopics LLC, Sherman Oaks, CA, United States, 4Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Multimodal MR neuroimaging was used to identify brain differences between attention deficit hyperactivity disorder (ADHD) associated with prenatal alcohol exposure (PAE) (ADHD+PAE), ADHD not associated PAE (ADHD-PAE) and normally-developing controls in an study of children aged 8-12 years. This is an interim report of findings obtained from an ongoing study after successful imaging of 73 subjects. Statistically-significant lateralized brain differences were identified in frontal white matter and gray matter. The findings provide preliminary support for the hypothesis that an objective diagnostic neuroimaging-based classifier can be developed.
Introduction
Prenatal alcohol exposure (PAE) leads to a substantial public health burden. Children with PAE are diagnosed with attention deficit hyperactivity disorder (ADHD) with high prevalence. Thereby, their ADHD is frequently falsely assumed to be idiopathic. This is unfortunate, as stimulant drugs, the most common ADHD treatment, are not as effective for ADHD related to PAE (ADHD+PAE) as for ADHD without PAE (ADHD-PAE). Moreover, most investigations of ADHD do not screen for PAE. Hence, samples of ostensibly idiopathic ADHD patients may include occult PAE subjects. It is critical to differentiate between ADHD+PAE and ADHD-PAE to establish biomarkers and treatment pathways in order to reduce long term negative outcomes. To acquire insight into brain differences between ADHD+PAE and ADHD-PAE, the authors are engaged in an study that uses multimodal MR imaging to study children aged 8-12 years who have been diagnosed with ADHD+PAE, ADHD-PAE and normally developing control children (Figure 1). Interim findings from this ongoing study will be presented.Subjects & Methods
Inclusion Criteria (All Groups): 1) age 8-12 yrs 2) IQ 70-115 (Wechsler Abbreviated Scale of Intelligence 3) English fluency.Exclusions: 1) co-occurrence of a known ADHD-associated genetic syndrome 2) developmental disorder or intellectual disability 3) medical/neurologic illness (eg seizure disorder, head trauma) 4) claustrophobia 5) ferromagnetic metal.
Inclusion Criteria (ADHD+PAE or ADHD-PAE Groups): 1) DSM-5 criteria for ADHD (any subtype) by KSADS-PL and clinical interview 2) (medication-free) average score > 1.7 on the parent-rated and > 2.0 on the teacher-rated ADHD Combined Scale of the SNAP-IV and current (med-free) CGI-S score for ADHD symptoms >4. Both medicated and unmedicated subjects are included but subjects taking stimulants are asked not to take their medication on neuroimaging days.
Further Criteria for Inclusion in ADHD+PAE Group: Every subject is assessed for diagnostic features of Fetal Alcohol Syndrome (FAS) using the modified Institute of Medicine criteria according to updated guidelines, which cover four key diagnostic features of FAS: 1) growth retardation 2) the FAS facial phenotype, including short palpebral fissures, flat philtrum, and flat upper vermillion border 3) neurodevelopmental dysfunction and 4) gestational alcohol exposure.
Neuroimaging: This single center study acquires neuroimaging data using a single 3T Siemens Prisma-Fit MRI system. Three-dimensional (3D) T1-weighted imaging, 3D T2-weighted imaging and diffusion tensor imaging (DTI) are acquired using pulse sequences and parameters developed by the Human Connectome Project (HCP). Single slice 1H-MRSI is performed using a 16x16 spatial encoding grid with a STEAM MRSI pulse sequence (TR/TE =2000/20). The MRSI slice is positioned to optimally cover frontal white matter (anterior and superior coronal radiata in the left and right hemispheres (LACR.LSCR, RACR.RSCR) and midline frontal gray matter (MFGM) targeted at the anterior middle cingulate cortex (aMCC). Gamma amino butyric acid (GABA) is measured using a single voxel J-editing pulse sequence with the voxel positioned to overlap with the aMCC. All neuroimaging data undergo expert quality control (QC) review. Head motion was the cause of the majority QC failures. QC failures are excluded from this report. This presentation reports an interim analysis of results derived from QC-pass MRS and DTI studies from 73 subjects and GABA aMCC studies from 67 subjects. MRSI and DTI co-analysis was performed using software developed for the HCP together with purpose-developed in-house software written in IDL and Python. GABA assessment from the single voxel J-edited pulse sequence was performed with LCModel. All MRS findings presented herein have been corrected for CSF content obtained through FSL segmentation of the 3D T1w volume image data. The data reported herein have also been corrected for age differences between the three study groups.
Results
Figures 2 and 3 present key measurements from short-TE MRSI together with DTI measured parameters for white matter (Figure 2: RACR.RSCR, Figure 3: LACR.LSCR). The most prominent MRS finding was higher Cho in ADHD+PAE compared to ADHD-PAE (p=0.028). Cho was significantly higher only in right hemisphere WM (RACR.RSCR). FA was lower in ADHD+PAE than in ADHD-PAE for LACR.LSCR (p=0.003) and RACR.RSCR (p=0.03). Additionally in RACR.RSCR, MD and RD were higher (p=0.006, p=0.005) in ADHD+PAE compared to ADHD-PAE. Figure 4 presents key short-TE MRSI findings from MFGM. NAA was lower in ADHD+PAE compared to ADHD-PAE (p=0.004). No other statistically significant differences were apparent. Figure 5 presents aMCC J-spectral editing GABA results. In ADHD-PAE, aMCC GABA was lower compared with controls (p=0.017) but there was not a corresponding difference between ADHD+PAE and ADHD-PAE.Preliminary efforts to develop a receiver-operating characteristics (ROC) discriminator for ADHD+PAE versus ADHD-PAE based on Cho, Glu, FA, MD and RD from the RACR.RSCR gave a ROC area under the curve (AUC) of 0.805±0.084 (p=0.005).
Conclusions
Statistically significant lateralized differences between ADHD+PAE and ADHD-PAE were identified using short-TE MRSI in combination with DTI and J-spectral editing in a moderately large cohort of pediatric subjects. The identification of differences between ADHD+PAE and ADHD-PAE supports the hypothesis that PAE causes a unique ADHD manifestation. The finding of elevated Cho, elevated diffusivity and reduced FA in frontal white matter in ADHD+PAE in comparison with ADHD-PAE suggests delay of white matter myelin development and/or inflammatory response in ADHD+PAE. There is preliminary support for the hypothesis that a objective neuroimaging-based diagnostic classifier could be developed from these differences.Acknowledgements
Financial support from NIH/NIAAA: AA0023884References
Figures
Figure 1: This study combines diffusion tensor imaging (DTI), short-TE MRSI and GABA J-spectral editing to evaluate differences in frontal white matter and gray matter between subjects having ADHD+PAE, ADHD-PAE and controls.

Figure 2: Short TE MRSI and DTI findings from the RACR.RSCR

Figure 3: Short TE MRSI and DTI findings from the RACR.RSCR
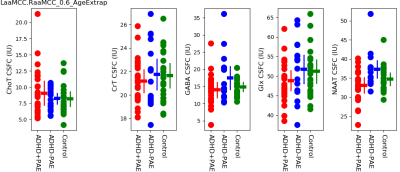
Figure 4: Short TE MRSI findings from MFGM
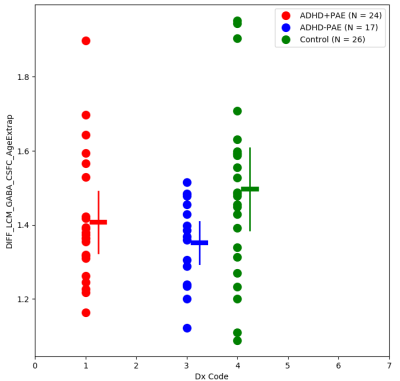
Figure 5: aMCC GABA results obtained by J-spectral editing