4579
White matter development after prenatal chemotherapy exposure: a diffusion MRI fixel-based analysis.1Oncology, KU Leuven, Leuven, Belgium, 2Imaging & Pathology, KU Leuven, Leuven, Belgium, 3Radiology, University Hospitals Leuven, Leuven, Belgium, 4Development and regeneration, KU Leuven, Leuven, Belgium, 5Gynaecology and Obstetrics, University Hospitals Leuven, Leuven, Belgium, 6Gynecological Oncology, The Netherlands Cancer Institute, Antoni van Leeuwenhoek, Amsterdam, Netherlands, 7Gynaecological oncology, Amsterdam university medical centers, Amsterdam, Netherlands
Synopsis
Currently, data are scarce on the long-term outcome of prenatal exposure to cancer treatment. In this preliminary analysis, we investigated white matter development in 9-year-old children who were prenatally exposed to chemotherapy, using multi-shell diffusion MRI and fixel-based analysis. We found indications of lower within-voxel (reflected by Fibre Density), macroscopic (reflected by Fibre Cross-section) and total (reflected by Fibre Density and Cross-section) intra-axonal volume of the splenium, isthmus and tapetal fibres of the corpus callosum in children with prenatal chemotherapy exposure compared to controls. This suggests prenatal chemotherapy exposure to potentially impact white matter development.
Introduction
The diagnosis of cancer during pregnancy poses difficult medical-ethical decisions, concerning both maternal and fetal health. While short-term outcomes after prenatal cancer treatment exposure are reassuring, long-term outcome data remain scarce1,2. In a recent event-related potentials study3, we demonstrated altered neural signaling after prenatal chemotherapy exposure in 9-year-old children, affecting executive functioning. This further emphasizes the need for long-term follow-up of children born after maternal cancer.In this preliminary analysis, we investigate white matter development in 9-year-old children who were prenatally exposed to chemotherapy, compared to healthy controls, using multi-shell diffusion MRI and fixel-based analysis.
Methods
Children in the prenatal-exposed group were born to mothers that were diagnosed with cancer during pregnancy and treated with chemotherapy after the first trimester of pregnancy. Children in the control group, born to healthy mothers, were matched on group level with regards to age, prematurity and gender. Children having major prenatal or neonatal complications known to possibly cause neurological sequelae, except for prematurity or intra-uterine growth restriction, were excluded. All children underwent MRI scanning at the age of nine years old, between 2015-2019, using the same scanner (3T Philips Achieva, 32-channel phased-array head coil).Echo-planar, Diffusion weighted MR images were obtained using b-values of 0,700 and 2000, acquired with respectively 6,30 and 60 uniformly distributed gradient directions. All volumes were acquired with the following set-up: resolution=2.5x2.5x2.5mm, FOV=240x240x125mm, TR/TE/FA=7000ms/72ms/90°, Phase encoding=AP, halfscan=0.766, SENSE=2 and total acquisition time=12:01min. Additionally, one b0 image was acquired with reversed phase-encoding.
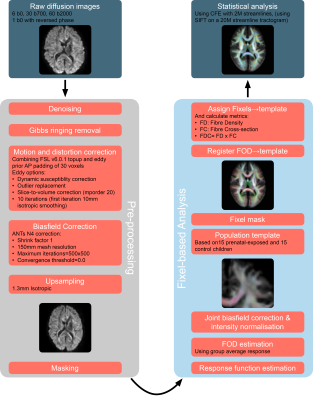
Diffusion images were analyzed with MRtrix4 (v3.0) following the recommended fixel-based analysis pipeline5, which was adapted for this dataset (Figure 1)6–8. Image quality was assessed by both visual inspection and quantitative reports generated using FSL’s automated quality control9.
Differences in fixel-based parameters of fibre density (FD), fibre cross-section (FC, logarithmically scaled) and their combined metric of fibre density and cross-section (FDC) were assessed at each individual fixel using non-parametric connectivity-based fixel enhancement10 (CFE), using 2 million streamlines. Fixels traversed by less than 150 streamlines were excluded from the final analysis. Group differences and effects of prematurity were tested with two general linear models. The first model only included group and normalized (z-score) gestational age at birth (GA, defined in days) as factors. In the second model, normalized Intracranial volume (ICV) was added as a covariate. ICV was calculated by back-transforming the group mask to subject-space, as described by Pannek et al.11 Significance was inferred at p<.05, corrected for multiple comparisons using Bonferroni correction
Results
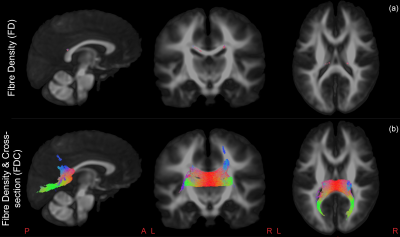
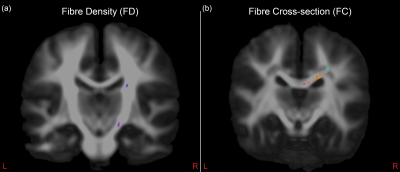
We included 22 prenatal-exposed children (11 boys, 13 born prematurely) and 39 healthy controls (20 boys, 21 born prematurely). Both groups did not significantly differ on GA, ICV or sex, as respectively tested using Mann-Whitney U-test or Fisher’s exact test. However, there was a small, though significant, difference in age between prenatal-exposed (median 9.24 years, range [9.01-9.94 years]) and control children (median 9.50 years, range [8.95-9.98years]).FDC was significantly lower in the prenatal-exposed group, bilaterally in the splenium, isthmus and the bilateral occipital, parietal and left temporal tapetal fibres of the corpus callosum (CC) (Figure 2:b). A smaller subset of bilateral fixels in the isthmus showed significantly smaller FD in the prenatal-exposed group (Figure 2:a). GA was significantly positively correlated with FD in a small set of fibres within the right cortico-spinal tract (CST) (Figure 3:a). FC was negatively correlated with GA in the right side of the splenium and parietal tapetal fibres of the CC (Figure 3:b). All results remained significant when corrected for ICV, except for FD with GA, which was marginally significant (p=.0506). Additionally, when corrected for ICV, FC was significantly lower bilaterally in the splenium of the CC of prenatal-exposed children compared to controls.
Discussion
The results of this study suggest that within-voxel (reflected by FD), macroscopic (reflected by FC) and total (reflected by FDC) intra-axonal volume of the splenium, isthmus and tapetal fibres of the CC to be lower in children who were prenatally exposed to chemotherapy, compared to controls. Previous research showed FD and FC in this region to be impacted in adult childhood cancer survivors treated with intravenous chemotherapy12. On the other hand, FD,FC and FDC were previously found to increase during prepubertal development13.GA positively correlated with FD in the right CST, while FC was found to negatively correlate with GA in the splenium of the CC. The decrease in FD corroborates earlier findings in literature on prematurity11, while the increase in FC is in line with literature on late-prematurity, where hypo- and hyper-connectivity is simultaneously observed14.
Conclusions
To our knowledge, this is the first study to investigate white matter development after prenatal chemotherapy exposure. These preliminary results suggest that prenatal chemotherapy exposure has an impact on WM development, which might include decreased axonal diameter and/or a lower axon count. Future work will include expansion of this dataset as well as linking DWI-derived parameters to clinical and psychological parameters.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation program(European Research council, grant no 647047), Foundation against cancer (Stichting tegen kanker, grant no. 2014-152) and Research Foundation Flanders (FWO, grant no. 11B9919N), J.B. is an aspirant researcher for the FWO, F.A. is a senior clinical researcher for the FWO. The computational resources and services used in this work were provided by the VSC (Flemish Supercomputer Center), funded by the FWO and the Flemish Government – department EWI.
The authors would like to thank: Jorine De Haan, Gunnar Naulaers, Charlotte Maggen, Liesbeth Leemans, Kaat Philippe, Cettina Schellens, Lara Stroobants, Tineke Vandenbroucke, Marie-Astrid Van Hoorick, Katrien Van Tornout, Dorothée Vercruysse, Magali Verheecke, Diane Wolput and all families participating in this study
References
1. Maggen C, van Gerwen M, Van Calsteren K, Vandenbroucke T, Amant F. Management of cancer during pregnancy and current evidence of obstetric, neonatal and pediatric outcome: a review article. Int J Gynecol Cancer. January 2019:ijgc-2018-000061. doi:10.1136/ijgc-2018-000061
2. de Haan J, Verheecke M, Van Calsteren K, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337-346. doi:10.1016/S1470-2045(18)30059-7
3. Blommaert J, Zink R, Deprez S, et al. Long-term impact of prenatal exposure to chemotherapy on executive functioning: An ERP study. Clin Neurophysiol. 2019. doi:10.1016/j.clinph.2019.06.012
4. Tournier J-D, Smith RE, Raffelt DA, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. bioRxiv. 2019. doi:10.1101/551739
5. Raffelt DA, Tournier JD, Smith RE, et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2017;144:58-73. doi:10.1016/j.neuroimage.2016.09.029
6. Andersson JLR, Graham MS, Drobnjak I, Zhang H, Campbell J. Susceptibility-induced distortion that varies due to motion: Correction in diffusion MR without acquiring additional data. Neuroimage. 2018;171:277-295. doi:10.1016/j.neuroimage.2017.12.040
7. Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556-572. doi:10.1016/j.neuroimage.2016.06.058
8. Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, Bastiani M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage. 2017;152:450-466. doi:10.1016/j.neuroimage.2017.02.085
9. Bastiani M, Cottaar M, Fitzgibbon SP, et al. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage. 2019;184(May 2018):801-812. doi:10.1016/j.neuroimage.2018.09.073
10. Raffelt DA, Smith RE, Ridgway GR, et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage. 2015;117:40-55. doi:10.1016/j.neuroimage.2015.05.039
11. Pannek K, Fripp J, George JM, et al. Fixel-based analysis reveals alterations is brain microstructure and macrostructure of preterm-born infants at term equivalent age. NeuroImage Clin. 2018;18(September 2017):51-59. doi:10.1016/j.nicl.2018.01.003
12. Sleurs C, Lemiere J, Christiaens D, et al. Advanced MR diffusion imaging and chemotherapy-related changes in cerebral white matter microstructure of survivors of childhood bone and soft tissue sarcoma? Hum Brain Mapp. 2018. doi:10.1002/hbm.24082
13. Genc S, Smith RE, Malpas CB, et al. Development of white matter fibre density and morphology over childhood: A longitudinal fixel-based analysis. Neuroimage. 2018;183(July):666-676. doi:10.1016/j.neuroimage.2018.08.043
14. Degnan AJ, Wisnowski JL, Choi S, et al. Altered structural and functional connectivity in late preterm preadolescence: An anatomic seed-based study of resting state networks related to the posteromedial and lateral parietal cortex. PLoS One. 2015;10(6):1-22. doi:10.1371/journal.pone.0130686
Figures