4577
Qualitative and Quantitative analysis of placental function to characterize pathophysiology in congenital heart disease (CHD)1Cardiovascular Imaging, King's College London, London, United Kingdom, 2Perinatal Imaging & Health, King's College London, London, United Kingdom
Synopsis
Congenital Heart Disease (CHD) is the most frequent congenital defect and varies widely in severity and subtype. The antenatal diagnosis of the severity is of key importance to plan delivery and treatment. The shared developmental pathways and close functional links with the fetal heart may lead to placental dysfunction that in turn may alter the in-utero environment to the detriment of mother and fetus. Hence, assessment of placental function delivers crucial complementary information to characterize pathophysiology in CHD. This study employs high-resolution whole-placental T2* relaxometry in 75 women (40 diagnosed with a fetus with CHD) to phenotype placental function.
Introduction
Congenital heart disease (CHD) is a common birth defect with a prevalence of up to 1% of all live births and the most frequent cause for in-utero mortality. The outcome varies widely depending on CHD type, therefore accurate early antenatal diagnosis is essential to plan delivery and required interventions in moderate and severe cases. Underlying origins are mostly considered multifactorial from genetic, epigenetic and environmental causes, but depend on specific CHD type. The placenta is the only fetomaternal organ responsible for all exchange of oxygen, carbon dioxide and nutrients, as well as essential immunological functions, accounting for its pivotal role in fetal development. Moreover the placenta and heart share similar developmental pathways and thus vulnerability to disturbances. Recent advances in fetal MRI allow for more sophisticated and quantitative analyses of placental oxygenation, mainly using T2* relaxometry [1,2,3], shown to correlate linearly with gestational age (GA) and to be predictive for low birth weight [1]. Using this method late reports also describe increased fetal cerebral blood oxygenation in response to Oxygen [4]. This study aims to investigate the use of functional in-vivo antenatal MRI to stratify the severity of placental alterations in presence of fetal CHD.Methods
75 women were recruited following informed consent as part of ongoing fetal studies at St Thomas’ Hospital. 35 were classed as healthy control singleton pregnancies after assessment of medical history, antenatal diagnosis and outcomes - no diagnosis of preeclampsia, maternal chronic hypertension, diabetes, birth <37 weeks or birthweight centile <10th or incomplete data, 40 cases were antenatally diagnosed with CHD (5 Hypoplastic left heart (HLH), 4 Tetralogy of Fallot (TOF), 3 Common arterial trunk (CAT), 13 aortic arch anomalies (right aortic arch, double aortic arch), 12 coarctation of the aorta (COA)) and 3 with other complex lesions (pulmonary atresia with major aortopulmonary collateral arteries, double outlet right ventricle, partial anomalous pulmonary venous drainage). Women were scanned in either supine or left lateral position in a 1.5T Philips Ingenia scanner using the 28-channel torso coil under clinical monitoring during the entire scan. A multi-echo gradient echo sequence covering the entire uterus in coronal orientation was acquired with a resolution of 2.5mm isotropic, FOV=360x360, 50-88 slices, no SENSE, no half-scan, TR=14s, TE=11.376 ms / 57.313 ms / 103.249 ms / 149.186 ms / 195.122 ms, free-breathing, TA=1min. T2* maps were calculated using monoexponential fitting in MATLAB and the whole placental ROI was manually segmented using ITK-SNAP by JS and JH. Mean T2*, lacunarity and whole placental volume was obtained and 2-way ANOVA performed. Thereby, included control data for statistical analysis was limited to the GA between 28 and 36 weeks to match the GA of the available CHD cases.Results
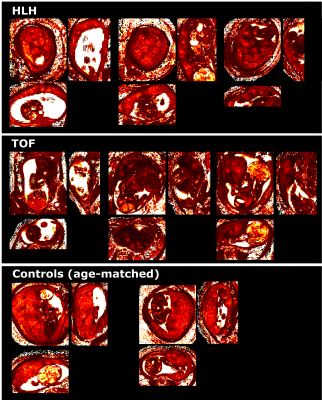
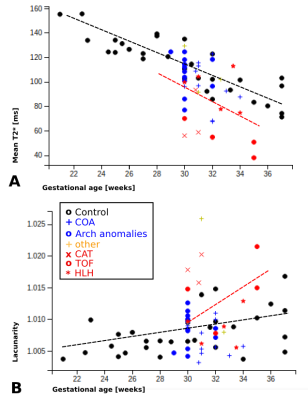
Placental T2* maps were successfully obtained from all participants, examples in HLH, TOF and age-matched controls are depicted in Fig. 1 with mid-parenchymal slices in all three orientations. Qualitative assessment shows most marked differences between TOF cases and controls, while HLH cases are only mildly different on visual inspection of the T2* maps. Quantitative results illustrate linear decay in mean T2* with GA by -4.45ms/week (p<0.00001), increasing lacunarity with GA (p=0.2) and increase in volume (p<0.01) (Fig. 2), in keeping with previous literature [1,5]. Preliminary results of the two-way ANOVA show decreased mean T2* over gestation for the CHD cases (p=0.5), increased volume (p=0.14) and increased lacunarity (p=0.4). However, a wide range of measured values among the CHD cases can be observed depending on CHD subtype. Mean T2* in cases of TOF appear lower without reaching statistical significance, while HLH cases appear to be appropriate in comparison to controls at matched gestational age. Cases of suspected COA appear to spread widely, reflecting the clinical spectrum of this lesion. Lacunarity in cases with antenatal diagnosis of CHD appears to trend upwards compared to controls without reaching statistical significance. To date, only 25 cases with antenatal CHD have delivered and neonatal outcome is available (52% female), median gestational age at birth was 38+4 (32+3 - 41+3), median birth weight 3080g (1610g - 5150g). 25 control cases have delivered and neonatal outcome is available (62% female), median gestation age at birth was 40+0 (36+4 - 42+1), median birth weight 3500g (2225g - 4450g). Full correlation of fetal findings to postnatal outcomes are anticipated once the entire cohort has been delivered.Conclusion
Placental T2* relaxometry can provide complementary information in the antenatal assessment of CHD. Results among the entire CHD cohort vary widely, but preliminary data suggests distinct phenotypes in certain subtypes, which warrant further evaluation once outcome and results from genetic testing will become available.Acknowledgements
The authors acknowledge all clinical and scanning team involved in these projects and are grateful to the patients and volunteers recruited for this project. This work was supported by Wellcome Trust IEH Award 102431 (iFIND project), the NIH Human Placenta Project grant 1U01HD087202-01 (Placenta Imaging Project (PIP)), the Wellcome Trust (Sir Henry Wellcome Fellowship, 201374/Z/16/Z), as well as the Wellcome EPSRC Centre for Medical Engineering at King;s College London (WT 203148/Z/16/Z) and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
[1] M. Sinding, D. A. Peters, J. B. Frøkjaer, O. B. Christiansen, A. Petersen, N. Uldbjerg, and A Sørensen. Placental magnetic resonance imaging T2* measurements in normal pregnancies and in those complicated by fetal growth restriction. Ultrasound in Obstetrics & Gynecology, 47(6):748–754, jun 2016. ISSN 09607692. doi: 10.1002/uog.14917.
[2] Matthias C. Schabel, Victoria H. J. Roberts, Jamie O. Lo, Sarah Platt, Kathleen A. Grant, Antonio E. Frias, and Christopher D. Kroenke. Functional imaging of the nonhuman primate Placenta with endogenous blood oxygen level-dependent contrast. Magnetic Resonance in Medicine, 76(5):1551–1562, nov 2016. doi: 10.1002/mrm.26052. URLhttp://www.ncbi.nlm.nih.gov/pubmed/26599502
[3] Jana Hutter, Paddy J. Slator, Laurence Jackson, Ana Dos Santos Gomes, Alison Ho, Lisa Story, Jonathan O’Muircheartaigh, Rui P. A. G. Teixeira, Lucy C. Chappell, Daniel C. Alexander, Mary A. Rutherford, and Joseph V. Hajnal. Multi-modal functional MRI to explore placental function over gestation.Magnetic Resonance in Medicine,sep 2018. doi: 10.1002/mrm.27447.
[4] Hemodynamic Responses of the Placenta and Brain to Maternal Hyperoxia in Fetuses with Congenital Heart Disease by Using Blood Oxygen–Level Dependent MRI, Wonsang You, Nickie N. Andescavage, Kushal Kapse, Mary T. Donofrio, Marni Jacobs, Catherine Limperopoulos, Radiology 2019
[5] André Ricardo Backes. A new approach to estimate lacunarity of texture images. Pattern Recognition Letters, 34(13):1455–1461, 2013. doi: 10.1016/j.patrec.2013.05.008.
Figures