4558
Quantitative evaluation of IVIM-DWI combined with simplified DKI for subtype diagnosis and grading prediction in retroperitoneal liposarcoma1radiology department, Shanghai public health clinical center, shanghai, China, 2General surgery, Shanghai public health clinical center, shanghai, China
Synopsis
This study used ivim-dwi combined with sDKI models to quantitatively evaluate the differential diagnosis and histological grading prediction of retroperitoneal liposarcoma subtypes. Our results indicated that IVIM-DWI combined with sDKI models could accurately differentiate partial subtypes of RPLS, which was helpful for clinical selection of the optimal treatment strategy.
Introduction
Intra-voxel incoherent motion diffusion-weighted imaging (IVIM-DWI) can quantitatively distinguish pure diffusion coefficient (D) and pseudo-diffusion coefficient (D*) caused by microcirculation or blood perfusion, which makes up for the deficiency of traditional DWI (1, 2). While diffusion kurtosis imaging (DKI) can more accurately describe the complex diffusion of water molecules in vivo and provide information about tissue heterogeneity and vascular composition (3). At present, IVIM-DWI and DKI are widely used in the differential diagnosis, grading prediction, efficacy monitoring and prognosis evaluation of systemic multiple organs and related diseases (4-7). However, the application of IVIM-DWI and DKI in retroperitoneal liposarcoma (RPLS) has rarely been reported. The purpose of this study was to explore the diagnostic value and predict the histological grading of RPLS by using IVIM-DWI combined with simplified DKI (sDKI) models.Materials and Methods
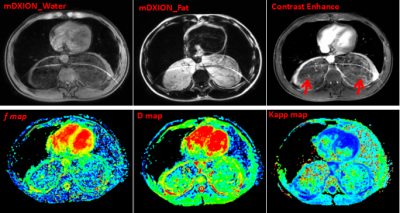
53 patients who were identified as RPLS by postoperative pathology underwent IVIM-DWI and sDKI scan. Among them, well-differentiated liposarcoma (WDLS, n=21 cases), dedifferentiated liposarcoma (DDLS, n=22 cases), myxoid liposarcoma (MLS, n=10 cases). These parameters of IVIM-DWI (f, D, D*, ADC) and sDKI (Dapp, Kapp) were measured by using the third-party post-processing software (IMAge/enGINE) (8). Histological grading of RPLS was evaluated by French Federation Natinale des centres de Lutte Cotre le Cancer(FNCLCC)classification system (9).Results
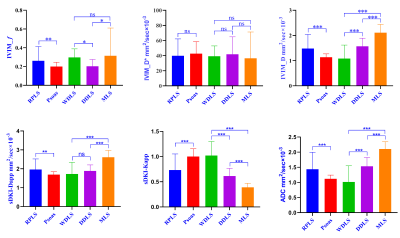
Mean D and ADC values of WDLS, DDLS and MLS groups increased successively, while Kapp values decreased successively, which were significant differences among three groups (p<0.001). Mean f values were significantly lower in DDLS group (0.20±0.07) than in WDLS (0.30±0.09, p=0.006) and MLS (0.32±0.30, p=0.009), whereas there was no significant difference between WDLS and MLS (p=0.681). Mean Dapp values were significantly higher in MLS group (2.61±0.36) than in WDLS (1.73±0.62, p<0.001) and DDLS (1.89±0.32, p<0.001), while no significant difference was found between WDLS and DDLS (p=0.135). D* value was no significant difference among the three groups (p>0.05). The area under curve (AUC) of f combined with Kapp in discriminating between WDLS and DDLS increased to 0.97 with sensitivity/specificity of 85.7%/100%; Kapp (AUC of 0.983, sensitivity/specificity of 90.5%/100%) had the highest accuracy in identifying between WDLS and MLS. The combination of Kapp and Dapp had the optimal diagnostic efficacy (AUC of 0.982, sensitivity/specificity of 100%/90%) in the differential diagnosis between DDLS and MLS. Spearman correlation analysis indicated the FNCLCC grading of RPLS had moderately negative correlations with f value (r=-0.423, p<0.01) and Kapp (r=-0.563, p<0.01); and moderately positive correlations with ADC (r=0.598, p<0.01) and D (r=0.611, p<0.01), but no correlation with Dapp (r=0.252, p=0.069).Conclusion
The quantitative parameters of IVIM-DWI combined with sDKI models are helpful to improve the accuracy and specificity in differential diagnosis of RPLS subtypes and have the potentiality to predict the histological grading of RPLS.Acknowledgements
Thanks for Philips' great support for this project, and thanks to the IMAge/enGINE research team (third-party post-processing software).References
1. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497-505.
2. Iima M, Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology. 2016;278(1):13-32.
3. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432-40.
4. Zhu L, Pan Z, Ma Q, Yang W, Shi H, Fu C, et al. Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology. 2017;284(1):66-76.
5. Ma W, Zhang G, Ren J, Pan Q, Wen D, Zhong J, et al. Quantitative parameters of intravoxel incoherent motion diffusion weighted imaging (IVIM-DWI): potential application in predicting pathological grades of pancreatic ductal adenocarcinoma. Quant Imaging Med Surg. 2018;8(3):301-10.
6. Meeus EM, Zarinabad N, Manias KA, Novak J, Rose HEL, Dehghani H, et al. Diffusion-weighted MRI and intravoxel incoherent motion model for diagnosis of pediatric solid abdominal tumors. J Magn Reson Imaging. 2018;47(6):1475-86.
7. Zheng X, Chen Y, Xiao Y, Zheng D, Chen W. Early diagnosis of radio-insensitive human nasopharyngeal carcinoma xenograft models by diffusion kurtosis imaging. Magn Reson Imaging. 2019;55:128-32.
8. Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37-42.
9. Yang M, Yan Y, Wang H. IMAge/enGINE: a freely available software for rapid computation of high-dimensional quantification. Quant Imaging Med Surg. 2019;9(2):210-8.
Figures