4551
A DTI Comparative study – Is demyelination in AD resembling primary demyelinating disease (MS) or secondary demyelinating disease (NPSLE)?1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Health Technology and Informatics, The Hong Kong Polytechnic University, Hong Kong, Hong Kong, 3Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, Hong Kong, 4Philips Healthcare, Hong Kong, Hong Kong, 5Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
Demyelination is a known pathology in AD, but it is unclear whether such myelin loss resembles primary or secondary demyelinating disease. Here, we retrospectively analysed the DTI data using voxel-wise TBSS analysis and made pair-wise comparisons between cohorts of AD, Relapsing-remitting MS(RRMS), NPSLE and normal controls (NC). In diffusion metrics analysis, widespread microstructural patterns were similarly found between AD and MS in terms of diffusion metrics, but not between AD and NPSLE. Our results indicate that the microstructural WM changes in AD may share similar pathological mechanisms in demyelinating disease of RRMS.
Introduction
The typical pathological findings of Alzheimer’s disease (AD) include senile plaques with amyloid β deposition, neurofibrillary tangles with tau accumulation and neuronal apoptosis1. Mounting research has shown changes in white matter (WM) regions2 and demyelination has been proposed to be a major pathogenetic mechanism in Alzheimer’s disease3. However, it remains poorly understood if AD resembles a primary demyelinating or a secondary demyelinating disease.Diffusion metrics of diffusion-tensor imaging (DTI) could be helpful for detecting the distinct mechanisms of white matter degeneration4, particular interest of which is radial diffusivity (RD), which measures the average magnitude of water diffusion perpendicular to the axonal fibres and is associated with myelin breakdown4.
We previously applied diffusion imaging to study retrogenesis in AD5, multiple sclerosis (MS) as a primary demyelinating disease6, and neuropsychiatric systemic lupus erythematosus (NPSLE) as a secondary demyelinating disease7. Based on these three separate cohorts, we compared the microstructural WM changes using the same DTI analysis technique to reveal the differences and similarities of the myelination abnormalities between AD, MS, and NPSLE.
Method
This study consisted of 67 participants: 17 AD patients, 17 Relapsing-Remitting MS (RRMS) patients, 19 NPSLE patients, and 14 cognitively normal controls (NC). All the subjects were retrospectively recruited from reports earlier5-7. Ethical approvals of the research protocol were granted by the Institutional Review Board of the University of Hong Kong and the Hospital Authority Hong Kong West Cluster. The demographic and clinical data were presented in Table 1.
DTI scan was performed in all the participants using a 3 T scanner (Achieva, Philips Healthcare, Best, The Netherlands). We used 32 gradient directions with b = 1000 s/mm2, and 1 direction with b=0 s/mm2.
DTI tract-based spatial statistics(TBSS) (adjusted for age and gender) was applied to compare white matter changes in the three cohorts (AD vs NC, RRMS vs NC, NPSLE vs NC) using Functional MRI of the Brain Software Library (FSL) version 6.0 (Analysis Group, FMRIB, Oxford, UK, http://fsl.fmrib.ox.ac.uk).
Result
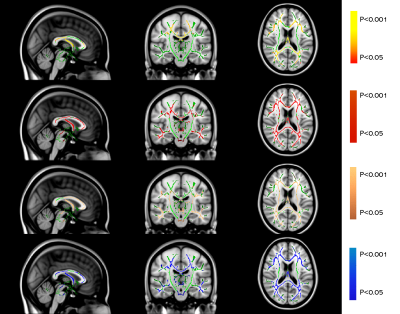
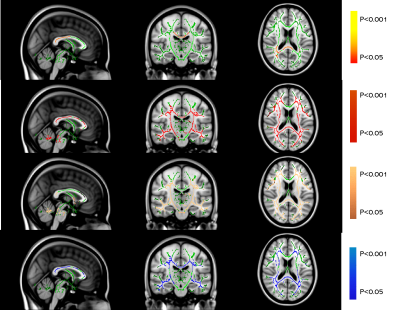
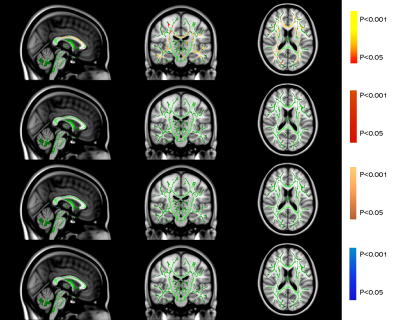
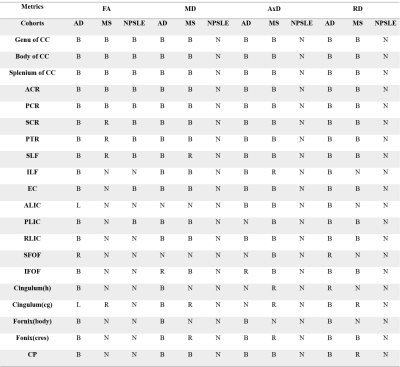
Both AD and MS showed extensive WM changes in four DTI-derived metrics, fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AxD) and radial diffusivity (RD), compared to NC (P<0.05, TFCE corrected for multiple comparison). But for NPSLE, significant WM changes were only found in FA, not in MD, AxD, and RD (Table 2 and Figure 1).
AD and RRMS versus NC
TBSS analysis (P<0.05, TFCE corrected for multiple comparison) revealed similar widespread microstructural abnormalities (in terms of MD, AxD, and RD) in brain regions including corpus callosum (CC), bilateral anterior corona radiata (ACR), superior corona radiata (SCR), posterior corona radiata (PCR), posterior thalamic radiation (PTR), superior longitudinal fasciculus (SLF), internal capsule(IC) and External capsule (EC) of AD and MS patients compared to NC (Table 2, Figure 1a and Figure 1b). But with respect to FA, WM degeneration was more severe in AD than MS compared to NC.
AD and NPSLE versus NC
AD seemed to have the same microstructural WM changes as compared to NPSLE in terms of FA, such as CC, bilaterally PTR, SCR, ACR, PCR and SLF (Table 2, Figure 1a and Figure 1c). But for MD, AxD and RD, NPSLE showed no significant change, while AD patients showed extensive microstructural WM abnormalities.
Discussion
FA quantifies the directional consistency of water diffusion along axonal tracts and is particularly sensitive to microstructural WM features, including myelination, axonal damage and cytoskeletal destruction4. In our study, AD shows similar pattern of diffusion metrics to RRMS, not NPSLE, which might suggest that myelin degradation in AD may resemble a primary demyelinating disease.RD measures the average magnitude of water diffusion perpendicular to the axonal fibers and is associated with myelin breakdown, namely demyelination4. Such an abnormality was evident in AD patients, which means that demyelination might play an important role in AD.
Conclusion
We found a similar pattern of microstructural WM changes between AD and RRMS, and our findings indicate that AD may share similar pathogenic mechanisms in primary demyelinating disease like RRMS.Acknowledgements
Health and Medical Fund, HKSAR and State Key Laboratory of Brain and Cognitive Sciences, HKU for funding support.References
1. Taoka T, Yasuno F, Morikawa M, et al. Diffusion tensor studies and voxel-based morphometry of the temporal lobe to determine the cognitive prognosis in cases of Alzheimer's disease and mild cognitive impairment: Do white matter changes precede gray matter changes? Springerplus 2016;5:1023.
2. Wang PN, Chou KH, Lirng JF, et al. Multiple diffusivities define white matter degeneration in amnestic mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 2012;30:423-437.
3. Tse KH, Herrup K. Re-imagining Alzheimer's disease - the diminishing importance of amyloid and a glimpse of what lies ahead. J Neurochem 2017;143:432-444.
4. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006;51:527-539.
5. Gao J, Cheung RT, Lee TM, et al. Possible retrogenesis observed with fiber tracking: an anteroposterior pattern of white matter disintegrity in normal aging and Alzheimer's disease. J Alzheimers Dis 2011;26:47-58.
6. Qian W, Chan KH, Hui ES, et al. Application of diffusional kurtosis imaging to detect occult brain damage in multiple sclerosis and neuromyelitis optica. NMR Biomed 2016;29:1536-1545.
7. Xu X, Hui ES, Mok MY, et al. Structural Brain Network Reorganization in Patients with Neuropsychiatric Systemic Lupus Erythematosus. AJNR Am J Neuroradiol 2017;38:64-70.
Figures