4547
Cerebrospinal fluid pulsation does not affect on DWI-based thermometry: healthy volunteer study1Kyoto Prefectural University of Medicine, Kyoto, Japan, 2Kyoto Prefectural University of Medicine Hospital, Kyoto, Japan
Synopsis
Diffusion-weighted imaging (DWI) based thermometry has the potential to be a non-invasive method of temperature measurement for the deep inside of human brain. Nevertheless, the DWI at lateral ventricle in brain might be influenced by the pulsation flow of cerebrospinal fluid (CSF), which is motivated by heartbeat. The purpose of this study was to investigate the influence of pulsation flow on brain DWI thermometry for healthy subjects. Comparisons were performed between magnetic resonance spectroscopy and DWI based thermometry (ΔT) at three CSF speed selections. There was no significant difference on ΔT among the CSF speed and volume on healthy subjects.
PURPOSE
Diffusion-weighted imaging (DWI) based thermometry has a potential to be a non-invasive method of temperature measurement for the deep inside of human brain [1-12]. DWI based thermometry uses apparent diffusion coefficient in lateral ventricle (LV) in brain. Nevertheless, the DWI at the LV might be influenced by the pulsation flow of cerebrospinal fluid (CSF), which occurs in sync with heartbeat. The purpose of this study was to investigate the influence of pulsation flow on brain DWI thermometry and compare to magnetic resonance spectroscopy (MRS) based thermometry for healthy subjects.MATERIALS AND METHODS
Subject: This study was approved by the ethics committee at our institution. Written informed consent for MR examinations were obtained from all subjects prior to participation in this study. A total of 70 healthy subjects (34 men, 36 women; mean (± standard deviation) age, 43.1 ± 15.4 years; range 21 - 71 years) voluntarily participated by leaflets displayed in our hospital.MRI acquisitions: All DWIs were obtained using a 3.0 T whole-body scanner (MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany). Single-shot echo-planar imaging was used for acquisition (repetition time, 3500 ms; echo time, 92 ms) with a motion-probing gradient in 10 orientations, b values of 1000 s/mm2, and averaging of two images. The field of view was 230 mm. A SENSE technique was used (128 × 53 data points) and reconstructed to 128 × 106 matrix (zero-filled, resolution of 128 × 128). A total of 45 slices with a thickness of 2 mm each were obtained without inter-slice gaps (trans-axial slices, parallel to AC-PC line). DWI data were used for assessing deep brain temperature at LV [2, 3].
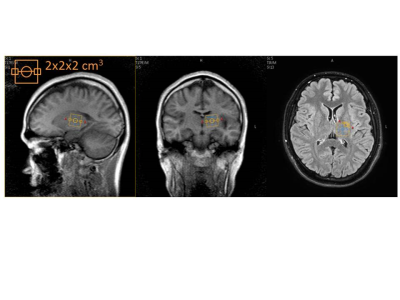
All MRS images also were obtained from a same 3.0 T whole-body scanner as DWI. A single-voxel region of interest (ROI) was manually placed at the left side of basal ganglia (Figure 1). Voxel size was 2 x 2 x 2 mm3. Acquisition of proton MR spectroscopy data was performed by using point resolved spectroscopy without water suppression. The following parameters were used: repetition time /echo time, 2000/40 msec; band width, 1200 Hz; and total acquisition time; 2.4 minutes.
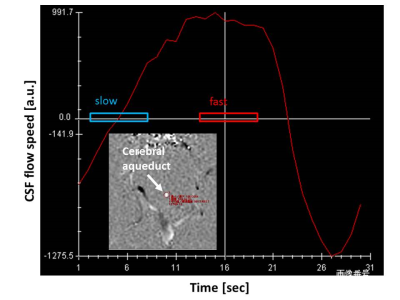
Heat Gating: The cardiac contraction was detected by peripheral pulse transducer. In addition, CSF pulsation flow at cerebral aqueduct was measured by phase contrast method and three DWIs were acquired at the timing of the maximum, the minimum, and at random ascending flow of CSF (Figure 2).
Temperature calculations: The diffusion coefficient along the direction of motion probing gradient D [mm2/s] was converted to temperature [13]; TDWI [°C] = 2256.74 / ln (4.39221 / D) - 273.15. Temperature within the LV and the mean temperature were calculated by histogram curve-fitting method [3]. The difference between brain temperature and MRS based temperature (ΔT = TDWI - TMRS) were used for the comparison.
MRS data was analyzed by using the automatic curve-fitting procedure, and decomposed into Lorenzian peak components by using custom software created in house by Matlab® (MathWorks®, Natick, MA, USA). The real part of the signal was used to estimate spectral parameters in a line shape fitting analysis. Temperature for each voxel was calculated from the chemical shift difference between water (H2O) and N-acethylaspartate (NAA) signals (ΔH2O - NAA) by using calibration data from Cady et al. [14]: TMRS [°C] = 286.9 - 94 (ΔH2O - NAA). MRS data were used for assessing deep gray matter temperature and treated as a gold standard.
Statistics: Comparisons were performed among ΔT at three CSF speed selections (slow vs. fast vs. random) by Student’s matched pair t-test (Matlab®). The correlation was evaluated as significant for P values < 0.05. The statistical power was 0.985 for this comparison (effect size = 0.5, G*Power 3.1.9.2, [15]).
RESULTS
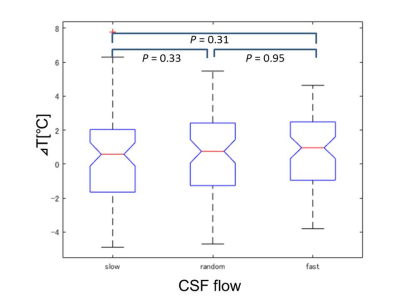
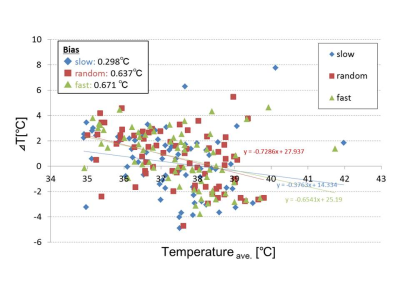
DWI thermometry along with pulsation: Figure 3 shows ΔT along the CSF flow speed at cerebral aqueduct. There was no significant difference among the CSF speed selections (P > 0.05).DWI thermometry vs. MRS thermometry: Figure 4 shows Bland-Altman plot between MRS and DWI based temperature. There were biases on DWI thermometry compared to MRS based temperature (slow = 0.298, random = 0.637, fast = 0.671°C). The slow CSF flow speed showed the lowest bias and proportional error.
DISCUSSIONS
Although no significant difference was observed between DWI thermometry by flow speed of CSF, observing the diffusion coefficient with slow speed by heart gating synchronization proved to be useful for DWI thermometry. There was a difference between individuals in the temperature of MRS and DWI based thermometry. It was about 0.2 °C on average, which was within the range of error, but some were observed as large differences within individuals. The reason was considered as follows. The proportion of the white and gray matter in ROI varies in each subject, which may also affect brain temperature measurement. The calibration formula for MRS thermometry was created with other MRI equipment and acquisition parameters. The DWI based temperature was also considered to include errors because the composition of CSF in LV is not pure water. A more detailed study is needed on the accuracy and limitations of human temperature measurements based on MRI.CONCLUSION
The CSF pulsation into the lateral ventricle during measurement of DWI does not significantly affect the measurement of DWI thermometry.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP17K10413 and JP17K10415.References
1. Yamada K, et al., Moyamoya patients exhibit higher brain temperatures than normal controls, NeuroReport, 2010; 21: 851-855.
2. Sakai K, et al., Age-dependent brain temperature decline as assessed by DWI thermometry, NMR in Biomedicine, 2011; 24(9): 1063-1067.
3. Sakai K, et al., Calculation methods for ventricular DWI thermometry: phantom and control studies, NMR in Biomedicine, 2012; 25(2): 340-346.
4. Sai A, et al., Diffusion-weighted imaging thermometry in multiple sclerosis, JMRI, 2014; 40(3): 649-654.
5. Ota M et al., Altered coupling of regional cerebral blood flow and brain temperature in schizophrenia compared with bipolar disorder and healthy subjects, Journal of Cerebral Blood Flow & Metabolism, 2014; 34(12): 1868-1873.
6. Tazoe J, et al., Brain core temperature of patients with mild traumatic brain injury as assessed by DWI-thermometry, Neuroradiology, 2014; 56(10): 809-815.
7. Sumida K, et al., Intraventricular cerebrospinal fluid temperature analysis using MR diffusion-weighted imaging thermometry in Parkinson’s disease patients, multiple system atrophy patients and healthy subjects, Brain and Behavior, 2015; 35: 5(6): e00340.
8. Kuriyama N, et al., Ventricular temperatures in idiopathic normal pressure hydrocephalus (iNPH) using DWI-based MR thermometry, Magnetic Resonance in Medical Sciences, 2015; 14(4): 305-312.
9. Tsukamoto T, et al., Assessment of brain temperatures during different phases of the menstrual cycle using diffusion-weighted imaging thermometry, Japanese Journal of Radiology, 2016: 34(4): 277-283.
10. Sumida K, et al., Intraventricular temperature measured by diffusion-weighted imaging compared with brain parenchymal temperature measured by magnetic resonance spectroscopy in vivo, NMR in Biomedicine, 2016; 29(7): 890-895.
11. Sparacia G, et al., Assessment of brain core temperature using MR DWI-thermometry in Alzheimer disease patients compared to healthy subjects, Japanese Journal of Radiology, 2017; 35: 168-171.
12. Sparacia G, et al., Brain core temperature of patients before and after orthotopic liver transplantation assessed by DWI-thermometry, Japanese Journal of Radiology, 2018; 36: 324-330.
13. Kozak LR et al., Using diffusion MRI for measuring the temperature of cerebrospinal fluid within the lateral ventricles, Acta Paediatr. 2010; 99(2): 237–243.
14. Cady EB et al., The estimation of local brain temperature by in vivo 1H magnetic resonance spectroscopy . Magn Reson Med 1995 ; 33 ( 6 ): 862 – 867 .
15. http://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower.html, accessed 5 Nov. 2019.