4543
Diffusion MRI revealed mild optic nerve fiber degeneration during Chimpanzee aging1Yerkes Imaging Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States, 2Department of Pediatric, Emory University School of Medicine, Atlanta, GA, United States, 3Division of Neuroscience and Neurological diseases, Emory University, Atlanta, GA, United States, 4Department of bioengineering, University of California, Riverside, Riverside, CA, United States
Synopsis
Aging effects on the optic nerve (ON) bundles of chimpanzees were investigated systematically with diffusion tensor imaging (DTI). Mean diffusivity (MD), axial diffusivity (AD or λll), radial diffusivity (RD or λτ) and fractional anisotropy (FA) showed mild and proportional changes from youth to elder adulthood, and significant increase of MD and RD were seen in elder chimpanzees. However, the changes are much milder than previous studies in the ON of monkey and human brain white matter. The unique evolution pattern in chimpanzee white mater aging may grant further investigations to unveil the neural substrate mechanism of the human brain aging.
Introduction
Chimpanzee is the closest relative to humans. Prior diffusion MRI studies indicated the brain white matter of chimpanzees degenerated differently from monkeys and humans during aging. Brain white matter is composed of large amount of bundles with branching and crossing fibers. In comparison, optic nerve (ON) is a more uniformed fiber bundle connecting directly the eye to the brain, allowing for further examination of aging effects on white matter. Therefore, examination of ON in a lifespan may reveal how the fiber bundle degenerate over time for knowledge on the white matter degeneration in brain aging. Diffusion tensor imaging (DTI) has demonstrated its robustness and sensitivity in several studies of the ON development and related pathologies in human and animals [1-3]. In the present study, the ON in chimpanzees from youth to late adulthood was investigated with DTI systematically.Materials and Methods
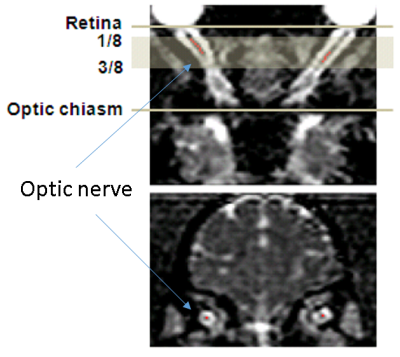
30 adult female chimpanzees (13 to 54 years old) were scanned with a diffusion-weighted, single-shot, double spin-echo EPI sequence on a Siemens 3T Trio scanner with the Siemens HE head coil. The imaging parameters were: TR = 5900 ms/TE = 86 ms, FOV = 130×230 mm2, matrix size = 72×128, voxel resolution = 1.8×1.8×1.8 mm3, 60 diffusion directions, b-value=0, 1000s/mm2. During MRI scans, animals were immobilized with cushions under anesthesia (1-1.5% isoflurane). End-tidal-CO2, inhaled CO2, O2 saturation, blood pressure, heart rate, respiration rate, and body temperature of animals were monitored continuously. DTI data were preprocessed with distortion and motion correction using FSL. All the DTI index maps were interpolated with customer-built Matlab codes and resliced with the software Analyze 9.0. Region of interests (ROIs) were selected manually using the software MRIcro on the ON slices located between 1/8 and 1/2 of the ON length, only the central voxel of the ON cross-section was selected on each slice, as shown in Fig. 1. Mean values of mean diffusivity (MD), axial diffusivity (AD orλ‖), radial diffusivity (RD or λτ) and fractional anisotropy (FA) in the ROIs were calculated for each ON from each animal. For analysis, the animals were divided into 3 groups: 13-20(n=13), 22-38(n=10), and 40-56 (n=7) years old. One-way ANOVA and Pearson correlation were used for statistical analyses.Results
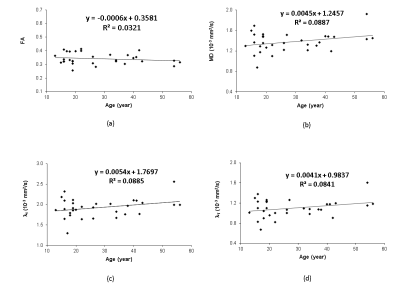
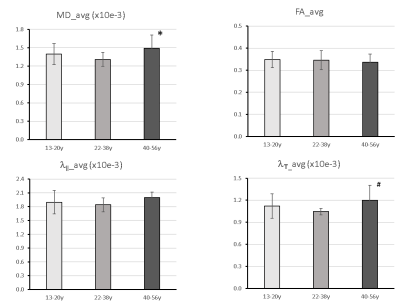
Progressive changes in the DTI indices (including MD, AD, RD and FA) of the ON of chimpanzees are illustrated in Fig. 2. The tendency of FA decrease and MD (and AD, RD) increase is seen but not significantly correlated with ages. However, the group analysis results show significant increase of MD and RD in elder chimpanzees ( Fig 3A and D).Discussion and Conclusion
Cognitive decline is generally seen in normal aging humans, but the causes of the decline remains poorly understood. Volumetric changes in cerebral cortex and microstructural changes (including demyelination and axonal loss) in brain white matter have been reported in human and animal studies of aging. Disrupted myelin and axon loss are seen in aged rhesus monkey brains by light and electron microscopy[4] , and in good agreement with previous ON studies of aged rhesus monkeys with DTI [5] and light and electron microscopy[6]. In comparison, the present DTI results revealed that the chimpanzee ON fibers do not degenerate aggressively as seen in macaques during aging but still show proportional evolution over the life span, in agreement with previous report of the brain white matter DTI study of the same cohort of chimpanzees[7]. As radial diffusivity is associated with demyelination and remyelination, the mild but significant MD and RD increase in the ON of aged chimpanzees suggest ON fiber’s slight demyelination but no axonal loss during aging. Also, compared to human brain DTI results, the changes of the DTI indices in chimpanzees are much milder [7]. Obviously, the present findings of species difference in primates suggest chimpanzees have unique evolution pattern in brain white matter aging. As the average lifespan of chimpanzees in captivity is 40-50 years, much shorter than humans (~80 years) but longer than macaque monkeys in cavities (~25 years), such unique evolution pattern in chimpanzee white mater aging may grant further investigations to unveil the neural substrate mechanism of the human brain aging by using advanced diffusion MRI techniques.Acknowledgements
No acknowledgement found.References
[1] Wheeler-Kingshott et al, MRM, 2006, 56:446-451.
[2] Xu et al, NMR Biomed, 2008, 21(9):928-940
[3] Yan et al, Int J Dev Neurosci. 2014 Feb;32:64-8
[4] Samdell and Peters, J Comp Neurol 2003, 466(1):14-30
[5] Yan, et al, Quant Imaging Med Surg. 2014 Feb;4(1):43-9. [
6] Sandell and Peters, J Comp Neurology, 2001, 429:541-553
[7] Chen et al, Neurobial Aging, 2013.34(10):2248-60.
Figures