4537
Brain white matter abnormalities and correlation with severity in amyotrophic lateral sclerosis: An atlas-based diffusion tensor imaging study1Fujian Medical University Union Hospital, Fuzhou, China, 2SIEMENS Healthcare, Shanghai, China
Synopsis
This study used atlas-based region of interest analysis to assess WM microstructure in ALS by combining intra-voxel metrics, which included FA and MD, and an inter-voxel metric, i.e., LDH. We found WM abnormalities extending from the motor to the extra-motor regions in ALS and observed a correlation between distinct diffusion metrics and various clinical variables, supporting DTI metrics may be utilized as the diagnostic biomarker of ALS. Meanwhile, the extent of WM abnormalities in several tracts (e.g. ATR and LIFOF) were better revealed by LDH measurement, suggesting the supplementary role in reflecting ALS-related pathological process.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease1. Many studies have used the intra-voxel diffusion metrics, such as fractional anisotropy (FA) and mean diffusivity (MD), to assess white matter (WM) pathology of ALS. Whereas another inter-voxel metric, called local diffusion homogeneity (LDH), has not been used for evaluating microstructural abnormalities in ALS. This study simultaneously utilized both intra- and inter-voxel diffusion metrics to more extensively assess microstructural changes in the WM of ALS, as well as analyze their correlation with clinical parameters.Methods
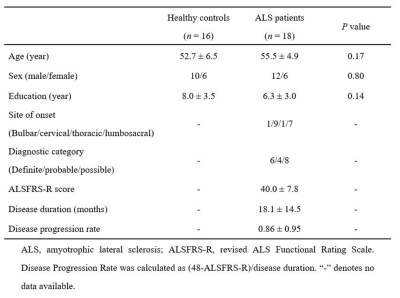
The 18 patients with ALS (1 familial, 17 sporadic) and 16 healthy controls were included in this study. ALS was diagnosed using the El Escorial criteria2, and disease severity was assessed using the revised ALS Functional Rating Scale (ALSFRS-R). The clinical and demographic data are provided in Fig. 1. MRI scans were performed on a 3.0T MR scanner. The DTI data were generated using a spin-echo single-shot echo-planar imaging sequence using the following parameters: b= 1000s/mm2 and 64 encoding diffusion directions; one non-diffusion-weighted image (b=0s/mm2 ); repetition time =2500 ms; echo time= 81ms; number of averages =1; slice thickness = 2mm without gap; field of view =260 mm× 260mm; matrix =130 ×130; flip angle= 90°; 72 axial slices; multiband factor =4.Diffusion-tensor models were calculated at each voxel and the FA, MD, and LDH maps were computed for each subject, implemented in the PANDA toolbox3. The atlas-based ROI (region of interest) analysis was performed to investigate the between-group difference in DTI measurements. The FA images of all subjects were aligned onto a FMRIB-58 FA template in the Montreal Neurological Institute (MNI) space with a non-linear registration algorithm, which was also employed in the MD and LDH images. Then, all images were smoothened using a 6-mm FWHM (Full Width at Half Maximum) Gaussian kernel. After that, the mean DTI metric values were extracted for each ROI.
The two-sample t-test was used to examine the between-group difference in every DTI measurement; Spearman correlation coefficients were computed to assess the correlation of clinical variables to the mean values of diffusivity parameters of the regions that survived between-group comparisons. And we conducted ROC analysis to assess the discrimination performance of various diffusion metrics between the two groups.
Result
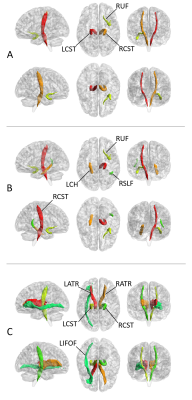
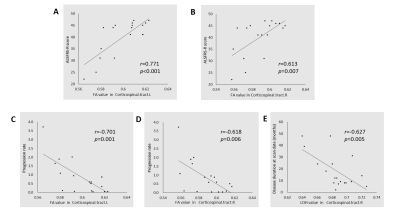
WM regions with diffusion metrics exhibiting significant between-group differences are presented in Fig. 2. Relative to the control group, the ALS group showed significantly lower FA values in the bilateral corticospinal tract and right uncinate fasciculus. The areas with higher MD were located in right corticospinal tract, left cingulum hippocampus, right uncinate fasciculus, and right superior longitudinal fasciculus (temporal part). In addition, ALS patients showed decreased LDH in bilateral anterior thalamic radiation (ART), bilateral corticospinal tract, and left inferior frontal-occipital fasciculus (LIFOF).Correlation analysis revealed a positive correlation between the mean FA values along the bilateral corticospinal tracts and the ALSFRS-R scores and a negative correlation between the mean FA values along the bilateral corticospinal tracts and the progression rate of the ASL patients (Fig. 3). Meanwhile, the LDH value along right corticospinal tract was negatively correlated with disease duration. No significant correlation was detected between various clinical measures and the MD values along the fiber tracts showing significant between-group differences.
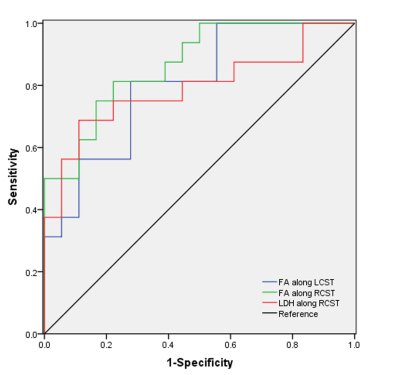
Furthermore, the WM tracts whose diffusion metrics exhibited significant correlations with clinical parameters were utilized as target tracts for ROC analysis (Fig. 4). FA value along right corticospinal tract (AUC =0.868, p=.003) and left corticospinal tract (AUC =0.802, p= .0003) showed moderate discrimination potential. Meanwhile, LDH values along right corticospinal tract (AUC = 0.792, p= .004) also exhibited moderate potential in distinguishing the two groups.
Discussion and conclusion
This study showed that ALS involves both intra-voxel and inter-voxel diffusion alterations across major tracts of the WM, which in turn is indicative of the contribution of WM microstructural changes to its pathogenesis. The findings indicate that DTI metrics may be utilized as indicators of neuropathological symptoms of ALS. In addition, the extent of abnormalities in several WM tracts such as ATR and LIFOF could be better assessed using the inter-voxel diffusion measurement; thereby, LDH can provide valuable information complementary to those obtained by conventional DTI metrics, when exploring WM alterations in ALS. Future studies should be performed to further clarify the pathophysiology processes related to diffusion measurement changes in ALS and to better evaluate the correlation between the diffusion parameters and the clinical symptoms or scales.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81501450), Fujian Provincial Science Fund for Distinguished Young Scholars (No. 2018 J06023), Fujian Provincial Program for Distinguished Young Scholars (No. 2017B023), Fujian Provincial Health Commission Project for Scientific Research Talents (No. 2018-ZQN-28), and Startup Fund for scientific research in Fujian Medical University(No.2018QH103).References
1. O. Hardiman, A. Al-Chalabi, A. Chio, E.M. Corr, G. Logroscino, W. Robberecht, P.J. Shaw, Z. Simmons, L.H. van den Berg, Amyotrophic lateral sclerosis, Nat Rev Dis Primers 3 (2017) 17071.
2. B.R. Brooks, R.G. Miller, M. Swash, T.L. Munsat, D. World Federation of Neurology Research Group on Motor Neuron, El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis, Amyotroph Lateral Scler Other Motor Neuron Disord. 1 (5) (2000) 293-299.
3. Z. Cui, S. Zhong, P. Xu, Y. He, G. Gong, PANDA: a pipeline toolbox for analyzing brain diffusion images, Front. Hum. Neurosci. 7 (2013) 42.
Figures