4536
White matter fiber density and cross-section alterations in neuropathic pain after spinal cord injury1Biomedical Engineering, University of Utah, Salt Lake City, UT, United States, 2Scientific Computing and Imaging Institute, University of Utah, Salt Lake City, UT, United States, 3Neurology, Neurosurgery, and Psychiatry, University of Utah, Salt Lake City, UT, United States
Synopsis
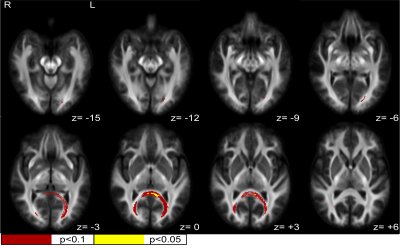
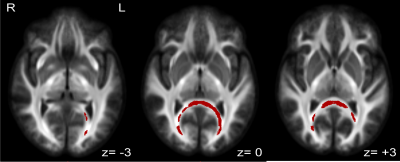
Fiber-specific white matter changes were compared using fixel-based analysis between spinal cord injured subjects with chronic neuropathic pain (n=17) and spinal cord injured subjects without any pain symptoms (n=15). Fiber density, fiber cross-section (FC), and a combined measurement of fiber density and cross section (FDC) were calculated and compared between groups using multi-shell, 3-tissue constrained spherical deconvolution and connectivity-based fixel enhancement. Statistically significant increases (FWE-corrected p<0.1) in FC and FDC were identified in a posterior-inferior commissural white matter pathway, corresponding to the splenium and major forceps of the corpus callosum and regions of the retrosplenial complex.
Introduction
Neuropathic pain is an idiopathic, chronic pain condition estimated to affect 50-60% of the spinal cord injured (SCI) population1. Current treatment methods are limited and mechanistic understanding of the development of neuropathic pain in the SCI population is lacking. Recent evidence suggests that subpopulations of neuropathic pain can be characterized based on neurophysiological signatures, including structural reorganization evident in structural and diffusion weighted neuroimaging2-4. This study aims to identify fiber-specific white matter changes specific to neuropathic pain following traumatic spinal cord injury. Identification of such neural biomarkers will provide insight into reactionary mechanisms in the brain and allow for the development of improved patient assessment and treatment.Methods
We recruited 32 subjects (7f), age 18-45 who had sustained a motor complete SCI at least one year prior to enrollment and collected demographic, pain symptoms and severity, anxiety, depression, and medical history information via questionnaire. 17 subjects reported moderate to severe chronic neuropathic pain (NP group) and 15 lacked any pain symptoms (control group). Multi-shell diffusion weighted MRI with 187 directions and 1.5mm isotropic voxels was collected for each subject using a 3T Siemens PRISMA system. Initial pre-processing of the imaging included motion and distortion correction using FSL5-8. MRtrix3 was used for bias field corrections, intensity normalization and all subsequent fixel-based analysis steps9. Fiber orientation distribution maps were generated using multi-shell, 3-tissue constrained spherical deconvolution to assess fiber bundle density and cross section differences between groups10. Fiber density (FD), fiber cross-section (FC), and a combined measure of fiber density and cross-section (FDC) were calculated across all white matter fixels for each subject. Fixelwise statistical comparisons of FD, FC, and FDC were compared between groups using connectivity-based fixel enhancement, with age, sex, and time since SCI as nuisance covariates11. Family-wise error (FWE) corrected p-value maps were then generated for each metric to show fiber tracts with significant differences between NP and control groups. Due to the small sample size of this study, significance was defined as p<0.1, though fixels significant at p<0.05 were also visualized for comparison.Results
There were no significant differences between the NP and control groups in any demographic measurement collected, except marital status, for which the NP group had a larger proportion of subjects who were married. Further, NP subjects showed significantly increased levels of anxiety and depression compared to controls. NP subjects rated their neuropathic pain symptoms as 5.7/10(+/- 1.94 STD) on average, based on the maximum subscore for the Neuropathic Pain Symptom Inventory for each subject. General pain severity and interference were 3.9/10(+/- 1.83 STD) and 3.6/10(+/- 2.85 STD), respectively, based on responses to the Brief Pain Inventory. Significant increases in FC and FDC were seen in NP patients in the posterior region of the splenium of the corpus callosum and retrosplenial complex region. At FWE-corrected p<0.05, these differences are only observed for FC in a small, more medial region within these areas. However, including p-values up to 0.1 elucidates a clear commissural pathway indicating differences in FC and FDC between NP and control groups from the splenium to the major forceps of the corpus callosum, again bordering on the retrosplenial complex, posterior cingulate cortex, and fusiform. Figure 1 shows a summary of fixels with significant increases in FC in NP when compared to controls at both p<0.05 and p<0.1 significance levels. Figure 2 shows fixels with p<0.1 significant increases in FDC. There were no significant differences between groups in FD at either p<0.1 or p<0.05.Discussion
NP subjects were shown to have significant increases in FC and FDC in a posterior-inferior commissural white matter pathway. We decreased the threshold for significance in this study for two reasons. The first is the small sample size of subjects relative to the statistical power for analysis of approximately 500,000 fixels. The second is the clear evidence of a white matter pathway with a conservative increase in threshold. We are confident that the observed results are consistent with a real difference between NP and control groups, because there were not significant fixels outside of the primary observed white matter pathway. Fibers in this pathway are likely to include and project to many brain regions including the retrosplenial complex, fusiform, hippocampi, occipital, and temporal lobes. Given that the only major difference between subject groups is the presence or absence of neuropathic pain symptoms, it is unlikely that the observed differences are due to visual, episodic, or oto-vestibular changes in white matter fibers commonly associated with this pathway12. However, studies have also implicated pathways in this region to emotional integration and response, interoception, proprioception, response to internal stimuli, and sensory integration12-13, which are much more likely to be responsive to chronic pain symptoms.Conclusion
Fiber pathways showing increases in FC and FDC in neuropathic pain subjects are likely to be involved in emotional, interoceptive, proproprioceptive, and sensory integration, however additional research is needed for any mechanistic interpretation to be made. Future work will include multi-modal neuroimaging analyses aimed at identifying correlated macro-structural and functional neuroimaging biomarkers, in order to develop a more comprehensive understanding of neuropathic pain after SCI.Acknowledgements
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1747505. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
This work was partially funded by the University of Utah Neuroscience Initiative, “Differentiating Neural Circuits Modulated During Therapeutic Versus Ineffective Deep Brain Stimulation". Principal Investigator: Christopher R. Butson PhD
The support and resources from the Center for High Performance Computing at the University of Utah are gratefully acknowledged. The computational resources used were partially funded by the NIH Shared Instrumentation Grant 1S10OD021644-01A1.
References
1. Burke, D., Fullen, B. M., Stokes, D. & Lennon, O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur. J. Pain 21, 29–44 (2017).
2. Lefaucheur, J.-P. Cortical neurostimulation for neuropathic pain. Pain (2016). doi:10.1097/j.pain.0000000000000401
3. Kruger, L. & Light, A. R. Translational pain research : from mouse to man. (CRC Press/Taylor & Francis, 2010).
4. Apkarian, A. V. A brain signature for acute pain. Trends in Cognitive Sciences (2013). doi:10.1016/j.tics.2013.05.001
5. M. Jenkinson, C.F. Beckmann, T.E. Behrens, M.W. Woolrich, S.M. Smith. FSL. NeuroImage, 62:782-90, 2012
6. Jesper L. R. Andersson and Stamatios N. Sotiropoulos. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125:1063-1078, 2016.
7. J.L.R. Andersson, S. Skare, J. Ashburner. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 20(2):870-888, 2003.
8. S.M. Smith, M. Jenkinson, M.W. Woolrich, C.F. Beckmann, T.E.J. Behrens, H. Johansen-Berg, P.R. Bannister, M. De Luca, I. Drobnjak, D.E. Flitney, R. Niazy, J. Saunders, J. Vickers, Y. Zhang, N. De Stefano, J.M. Brady, and P.M. Matthews. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(S1):208-219, 2004.
9. D.A. Raffelt, J.-D. Tournier, R.E. Smith, D.N. Vaughan, G. Jackson, G.R. Ridgway, A. Connelly. Investigating White Matter Fibre Density and Morphology using Fixel-Based Analysis. NeuroImage, 144 (2017), pp. 58-73.
10. T. Dhollander, D. Raffelt, and A. Connelly. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. ISMRM Workshop on Breaking the Barriers of Diffusion MRI (2016), pp. 5.
11. D.A. Raffelt, R.E. Smith, G.R. Ridgway, J.-D. Tournier, D.N. Vaughan, S. Rose, R. Henderson, A. Connelly. Connectivity-Based Fixel Enhancement: Whole-Brain Statistical Analysis of Diffusion MRI Measures in the Presence of Crossing Fibres. NeuroImage 117 (2015), pp. 40–55.
12. A Aralasmak, JL Ulmer, M Kocak, CV Salvan, AE Hillis, DM Yousem. Association, Commissural, and Projection Pathways and Their Functional Deficit Reported in Literature. Journal of Computer Assisted Tomography 30(2006), pp. 695-715.
13. R Maddock. The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences 22 (1999), pp. 310-316.
Figures