4531
Diffusion MRI reveals macro- and microstructural changes in cosmonauts' brains after long-duration spaceflight1Lab for Equilibrium Investigations and Aerospace, Dept. of Physics, University of Antwerp, Antwerp, Belgium, 2Translations Neuroscience, Dept. of Medicine, University of Antwerp, Antwerp, Belgium, 3Institute of Biomedical Problems, Russian Academy of Sciences, Moscow, Russian Federation, 4Dept. of Radiology, Federal Center of Treatment and Rehabilitation, Moscow, Russian Federation, 5Laboratory for Cognitive Research, National Research University Higher School of Economics, Moscow, Russian Federation, 6Dept. of Imaging and Pathology, KU Leuven, Leuven, Belgium, 7Dept. of Radiology, Royal Perth Hospital and University of Western Australia, Perth, Australia, 8Faculty of Fundamental Medicine, Lomonosov Moscow State University, Moscow, Russian Federation, 9Coma Science Group, Dept. of Neurology, University (Hospital) of Liège, Liège, Belgium, 10German Center for Vertigo and Balance Disorders, Dept. of Neurology, Ludwig-Maximilians-University Munich, Munich, Germany, 11imec - Vision Lab, Dept. of Physics, University of Antwerp, Antwerp, Belgium
Synopsis
There is currently limited information on the effects of spaceflight on the human brain. Therefore, we investigated longitudinal changes in gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) density and mass using diffusion MRI data acquired pre-flight, post-flight and at follow-up. Our results show redistributed CSF and concomitant GM morphological changes post-flight, which reverse at follow-up. At the same time, our results show no evidence of neurodegeneration. Moreover, GM and WM mass increased in sensorimotor brain regions post-flight, which largely persisted at follow-up. These results indicate, for the first time, sensorimotor neuroplasticity after spaceflight.
Introduction
Spaceflight is known to induce changes in many different physiological systems. One major factor causing these changes is the microgravity condition. Remarkably, few studies have addressed the question of how spaceflight affects the structure and function of the human brain. Most of the existing studies use voxel-based morphometry (VBM) of conventional anatomical MRI scans 1,2. VBM uses spatial information and priors to obtain discrete tissue segmentations, rather than measuring the continuous densities of each tissue type directly 3. As a result, VBM is sensitive to local volumetric changes, but does not provide insight into the tissue microstructure. For example, regarding the gray matter (GM) volume decreases observed in 1,2, it remains unclear whether these constitute a loss of tissue. In this work, we used multi-tissue constrained spherical deconvolution (MT-CSD) of diffusion MRI (dMRI) data 4 to directly estimate the apparent densities of white matter (WM), GM and cerebrospinal fluid (CSF) and investigated changes in these measures shortly after and seven months after spaceflight.Methods
Thirteen cosmonauts were scanned before (pre-flight) and on average 9 days after spaceflight (post-flight), where flight duration was on average 6 months. Nine subjects received an additional scan 7 months after spaceflight (follow-up). dMRI data were acquired on a 3T scanner using an advanced multi-shell acquisition scheme.Preprocessing included denoising 5, Gibbs-ringing suppression 6, susceptibility and Eddy current induced distortion correction 7, and bias field correction 8. MT-CSD with population-averaged tissue specific response functions was used to obtain the apparent densities of WM, GM- and CSF-like tissue 4. Spatial normalization was achieved by warping all tissue density maps to an unbiased study-specific template using a multi-level iterative nonlinear registration and averaging approach 9,10. Additionally, density maps were scaled by the determinant of the local Jacobian of the warps to obtain modulated density maps, which take into account local volume differences between scans, therefore reflecting the absolute tissue mass rather than the relative density.
Paired t-tests between time points were performed using a general linear model. Time between the date of landing and the dates of the post-flight and follow-up scans were used as nuisance variables. We applied threshold-free cluster enhancement 11 on the resulting t-statistic maps, after which we obtained p-values using non-parametric permutation testing with family-wise error correction.
Results
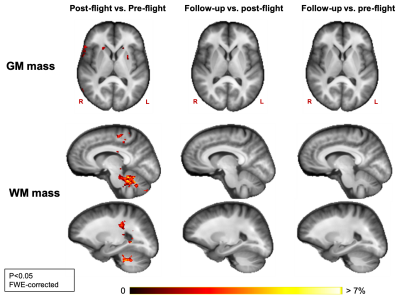
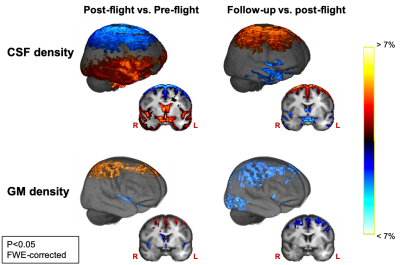
WM mass increased in the cerebellum, in parts of the corticospinal tract and just below the primary motor cortex between pre- and post-flight. GM mass increased from pre- to post-flight in the basal ganglia (Fig 1).GM density increased and CSF density and mass decreased from pre- to post-flight along the cortical gyri of the superior frontal and parietal lobes, with the largest effect below the vertex (Fig 2). Similarly, GM density was decreased and CSF density and mass was increased along the borders of the Sylvian fissure and along the ventricular borders (Fig 2). Additionally, CSF density and mass increased along a large area of the temporal, occipital and inferior frontal lobes (Fig 2).
When comparing post-flight to follow up, we found GM density decreases and CSF density and mass increases in the superior frontal and parietal lobes, highlighting a normalization of the post-flight effects (Fig 2). Along the Sylvian fissure and the ventricular borders, no significant change was observed in GM density when comparing post-flight to follow-up, whereas CSF density and mass did show some decrease. However, CSF mass remained significantly elevated at follow-up compared to pre-flight.
Discussion
Our results provide evidence of neuroplasticity occurring in several areas involved in sensorimotor processing, such as the cerebellum and the basal ganglia. The structural changes in these areas could reflect the necessary adjustments of the brain to allow appropriate motor function of cosmonauts in microgravity 12. Our results also show clear signs of a CSF redistribution as a result of spaceflight, which confirms earlier findings 1,13,14. Additionally, we find GM density alterations in the same regions where CSF changes were detected. We ascribe these results to the morphological effect of the intracranial fluid redistribution on the GM tissue. For example, decreased CSF in the superior frontal and parietal sulci induces a crowding of the cortical GM in this area, resulting in increased GM density estimates. As no GM mass changes were observed in these regions, we demonstrate that there is no change in the net amount of GM tissue. Therefore, the post-flight GM density decreases along the borders of the ventricles and Sylvian fissure do not appear to result from neurodegeneration, but rather from increased partial volume effect with CSF. This information resolves an important missing link from previous VBM studies, where GM volume decreases were observed after spaceflight, though without providing much information on the underlying mechanism.Conclusion
Our study provides evidence of neuroplasticity in sensorimotor brain areas after spaceflight. These changes are likely beneficial for astronauts’ optimal motor performance. The CSF redistribution and its partial normalization seven months after spaceflight must be followed up closely, as this effect is a likely cause of visual acuity impairment observed in a subset of astronauts. No signs of neural tissue damage were found in this study, which is a positive outcome for continuing human space travel in the future.Acknowledgements
No acknowledgement found.References
1. Van Ombergen, A. et al. Brain Tissue–Volume Changes in Cosmonauts. N. Engl. J. Med. 379, 1678–1680 (2018).
2. Koppelmans, V., Bloomberg, J. J., Mulavara, A. P. & Seidler, R. D. Brain structural plasticity with spaceflight. NPJ Microgravity 2, 2 (2016).
3. Ashburner, J. & Friston, K. J. Voxel-Based Morphometry—The Methods. NeuroImage 11, 805–821 (2000).
4. Jeurissen, B., Tournier, J.-D., Dhollander, T., Connelly, A. & Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103, 411–426 (2014).
5. Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. Neuroimage 142, 394–406 (2016).
6. Kellner, E., Dhital, B., Kiselev, V. G. & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 76, 1574–1581 (2016).
7. Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078 (2016).
8. Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).9. Raffelt, D. et al. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage 56, 1171–1180 (2011).
10. Pietsch, M. et al. A framework for multi-component analysis of diffusion MRI data over the neonatal period. Neuroimage 186, 321–337 (2019).
11. Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98 (2009).
12. Bostan, A. C. & Strick, P. L. The basal ganglia and the cerebellum: nodes in an integrated network. Nat. Rev. Neurosci. 19, 338–350 (2018).
13. Van Ombergen, A. et al. Brain ventricular volume changes induced by long-duration spaceflight. Proceedings of the National Academy of Sciences 116, 10531–10536 (2019).
14. Lee, J. K. et al. Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution. JAMA Neurol. 76, 412–419 (2019).
Figures