4526
Rich-Club Organizational Changes Over the Course of Motor Recovery after First-Time Acute Stroke1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, China
Synopsis
In this study we acquired diffusion data and motor functions from stroke patients at within 1 week, 1, 3 and 6 months and found rich-club organization remerged one month after stroke and high order regions were found at 6 months when patients functionally recovered. Feeder connections showed middle cost and high capacity while rich-club connections showed high cost and middle capacity at 6 months after stroke. Together these results suggest that there could be a potential relation between reemergence of rich-club organization and brain motor recovery.
Introduction
Numerous studies have demonstrated that the relation between brain changes and functional impairment and recovery should be studied from the perspective of brain network 1. Considering that there exists a group of brain regions with large number of connections, known as rich-club organization, that may govern brain functions 2–4, we hypothesize that such rich-club organization may be sensitive to brain motor recovery after stroke. In this study, we therefore aimed to investigate rich-club organizational changes over the course of motor recovery for patients with first-time acute ischemic stroke in the motor system.Methods
MRI experiments (3.0T Achieva TX, Philips, Best, Netherland) were performed and motor functions (upper-extremity Fugl-Meyer motor scale, UE-FM, and Barthel index, BI) were measured from n = 17 stroke patients (age: 44 – 84; infarct side: 33% left) at within 1 week, and 1, 3 and 6 months after stroke (n = 13, 16, 13, 9, respectively). DTI was performed using SS-SE-EPI with TR/TE = 4000/81 ms, b-value = 1000 s/mm2, 32 diffusion directions, and 3 mm isotropic resolution. The pipeline for the estimation of structural connectome (fiber count as weights) from DTI data is the same as that previously published 5. Group-averaged connectome was subsequently obtained for each time point (connections that existed in no more than 75% of all subjects were zeroed).To determine the existence of rich-club organization, rich-club coefficient of a subnetwork with a given threshold of nodal degree as compared to that of a random network with the same degree distribution and weights (permutation testing with n = 10,000) was computed 6,7. All brain regions were divided into rich-club and peripheral nodes. The local efficiency, local clustering coefficient and betweenness centrality of these two node types were measured. All brain connections were divided into rich-club (between rich-club nodes), feeder (between rich-club and peripheral nodes), and local (between peripheral nodes) connections 8. Network density (i.e. fiber count), network cost (product between fiber count and length), and communication cost (based on the topological distance between any brain region pair) were estimated 8. The cost/density ratio and communication cost/density ratio of three connection types were estimated to evaluate cost and capacity with imbalanced rich-club nodes.
Two-way ANOVA (Between subject factor: rich-club and peripheral nodes; or rich-club, feeder, and local connections. Time factor: time after stroke) with post-hoc analysis were performed. One-way repeated measures ANOVA was also performed to investigate the effect of time on motor recovery.
Results and Discussion
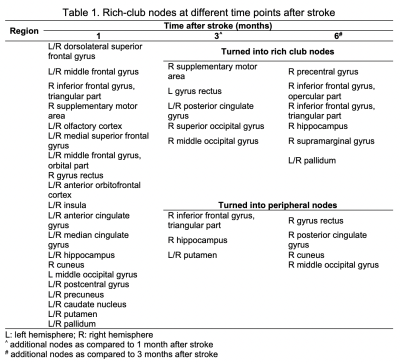
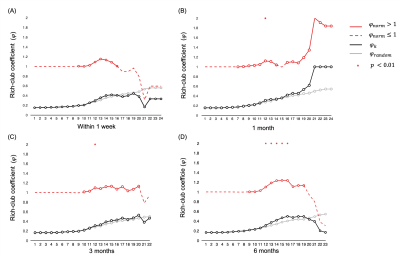
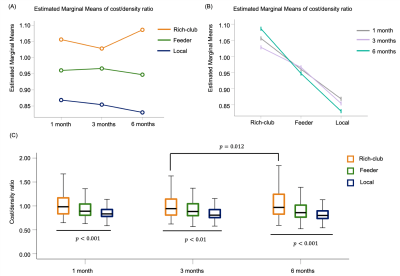
One-way repeated measures ANOVA showed that the BI (F = 20.20, p < 0.01) and UE-FM (F = 6.68, p = 0.0295) increased with the time after stroke. The BI measured at 3 and 6 months were higher than baseline at less than a week after stroke (p < 0.01 and < 0.001, respectively), whilst UE-FM were not (p = 0.1176 and 0.0936). Rich-club organization was found from the group-averaged structural brain network at all time points, except within a week after acute stroke (Figure 1). These rich-club nodes are tabulated in Table 1. These results showed that rich-club organization remerged one month after stroke. Higher order brain regions, those with larger nodal degree, contributed to part of the rich-club organization only at 6 months post stroke, at which point patients already functionally recovered.There was no interaction effect between time and node types on the local efficiency (F = 0.750, p = 0.473), local clustering coefficient (F = 0.782, p = 0.458) and betweenness centrality (F = 2.938, p = 0.055). There was a main effect of node type on local clustering and rich-club nodes had lower local clustering coefficient (F = 15.221, p < 0.001) compared with peripheral nodes. There was a main effect of time on betweenness centrality (F = 23.532, p < 0.001) and it decreased in the order - 1, 3 and 6 months after stroke. There was an interaction effect between time and connection types on the cost/density ratio (F = 2.801, p = 0.025). As shown in Figure 2, there was a simple main effect of time on the cost/density ratio of rich-club connections (F = 4.174, p = 0.015), whereby the cost/density ratio at 6 months post stroke was higher than 3 months (p = 0.012). For each time point, the cost/density ratio decreased in the order - rich-club (high), feeder (middle) and local (low) connections (p < 0.01).
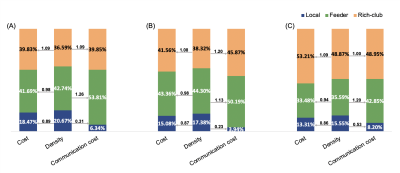
When examining all of the shortest communication paths (Figure 3), cost of rich-club connections accounted for an increasing percentage of total communication cost (from 39.85%, 45.87% to 48.95%), while feeder connections accounted for a decreasing percentage (from 53.81%, 50.19% to 42.85%). Both of rich-club (from 1.09, 1.20 to 1.00) and feeder connections (from 1.26, 1.13 to 1.20) showed a fluctuate capacity over the course of stroke recovery. Combining cost/density ratio, feeder connections showed a phenomenon of middle cost and high capacity while rich-club connections showed high cost and middle capacity when patients functionally recovered. These results are different from the high-cost and high-capacity of rich-club organizations of healthy controls as previously reported 8.
Conclusion
Our results suggest that there could be a potential relation between reemergence of rich-club organization and brain motor recovery.Acknowledgements
No acknowledgement found.References
1. Fornito, A., Zalesky, A., & Breakspear, M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16(3):159-172.
2. Van Den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31(44):15775–15786.
3. Senden M, Deco G, de Reus MA, Goebel R, van den Heuvel MP. Rich club organization supports a diverse set of functional network configurations. Neuroimage. 2014;96:174–182.
4. Collin G, Sporns O, Mandl RC, van den Heuvel MP. Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cereb Cortex. 2013;24(9):2258–2267.
5. Xu, X., Lau, K. K., Wong, Y. K., Mak, H. K., & Hui, E. S. The effect of the total small vessel disease burden on the structural brain network. Sci Rep. 2018;8(1):7442.
6. Colizza V, Flammini A, Serrano MA, Vespignani A. Detecting rich-club ordering in complex networks. Nat Phys. 2006;2(2):110.
7. Opsahl T, Colizza V, Panzarasa P, Ramasco JJ. Prominence and control: the weighted rich-club effect. Phys Rev Lett. 2008;101(16):168702.
8. Van den Heuvel MP, Kahn RS, Goñi J, Sporns O. High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci. 2012;109(28):11372–11377.
Figures