4525
Abnormal insula white matter tracts in smokers1Department of Radiology, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Synopsis
The insula, a cortical region that is thought to play a central role in this reward circuitry, has been implicated as an important role in the maintenance of nicotine addiction. However, it remains largely unclear about the alterations in white-matter tracts of insula circuits in nicotine addiction. Here, we further investigated the differences of insula white-matter tracts between smokers and nonsmokers. We found abnormal white matter tracts of insula subregions in smokers. These altered insula microstructural connectivity could interfere with the normal neural circuitry of reward processing, which might be the underlying neurobiology of nicotine addiction.
Purpose
Cigarette smoking, one of the biggest threats to human health, is responsible for 6 million preventable deaths annually1.Nicotine addiction is characterized as a neural circuit dysfunction, particularly with regard to the alterations in central reward pathways2, which can directly or indirectly contribute to compulsive seeking and taking of cigarette smoking. The insula, which is activated by smoking cues3, is thought to play a central role in this reward circuitry4. Damage to the insula disrupts smoking behavior in stroke patients and animal models5, which supports a critical role for the insula in the maintenance of nicotine addiction. Additionally, functional MRI (fMRI) studies have shown lower insula-based functional connectivity among smokers relative to nonsmokers6,7. However, it remains largely unknown about the alterations in white-matter diffusion properties of the insula tracts in smokers.In general, the insula structural circuits related to addiction are divided into two pathways: (1) the insula projects directly to the ventral striatum─the nucleus accumbens (NAc), a basal ganglia structure that is important for dopamine-mediated reward learning; and (2) the insula projects directly to the orbitofrontal cortex (OFC), which regulates striatal dopamine activation and the motivation to approach smoking. Based on the above priori knowledge, in this present study, we chose the NAc and the OFC as the regions of interest (ROIs) and employed diffusion tensor imaging (DTI) and probabilistic tractography to investigete the differences in white-matter connections of insula-to-NAc and insula-to-OFC tracts between smokers and nonsmokers. This could indicate altered neural connectivity and network function, affecting nicotine-reward processing.Methods
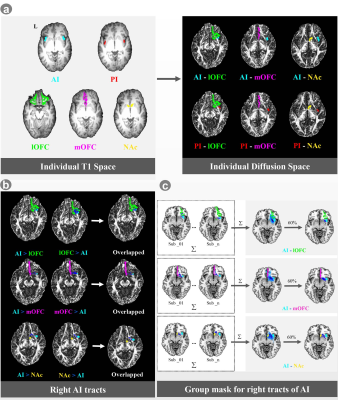
Nicotine-dependent smokers and nonsmoking controls were recruited in this study, all of whom underwent diffusion tensor imaging(DTI) scans. FreeSurfer (v5.3.0, https://surfer.nmr.mgh.harvard.edu/) provides an automated parcellation of anatomical regions of the cortices and subcortical regions in both hemispheres. Both anterior insula (AI) and posterior insula (PI) were extracted as seeds. Moreover, the OFC (including the lateral and the medial OFC (lOFC and mOFC)), and the NAc in both hemispheres were extracted as ROIs. DTI images were processed with FMRIB's Diffusion Toolbox 3.0 in FSL 5.0.9 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Before probabilistic tractography, all the ROIs masks (AI, PI, NAc, mOFC and lOFC) in the individiual T1 space were registered to the individiual diffusion space (Fig. 1a). The seed-based probabilistic tracking was performed between each seed (AI and PI) and each tagert (NAc, mOFC and lOFC) in each hemisphere. To ensure that only white-matter tracks were kept for calculating, each fiber-track was conducted twice, e.g., once using the AI as a seed mask, the ipsilateral NAc as a termination mask (AI_NAc tracts), and vice versa (i.e., NAc_AI tracts: NAc=seed mask; the ipsilateral AI=termination mask). For each voxel in the seed mask, 5000 times tracking was performed. For each subject, the AI_NAc and NAc_AI tracts were binarized and then overlapped in individual diffusion space (Fig. 1b). The final tract mask for each insula circuit was generated by keeping the voxels with values larger than 60% of the total number of the subjects in each group (Fig. 1c). Mean values for FA, axial (AD) , radial (RD), and mean diffusivity (MD) of identified tracts were extracted for statistical analysis. The other insula tracts were tracked similarly. Independent t-tests were used to compare demographic characteristics and DTI results between smokers and nonsmokers.Results
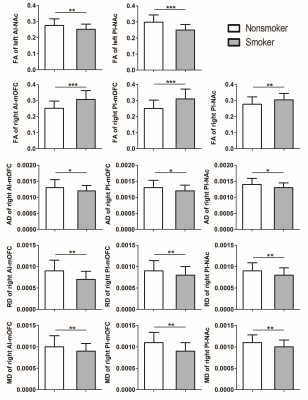
In total, 58 nicotine-dependent smokers and 34 matched nonsmoking controls were recruited in this study. There were no differences for age and education between smokers and nonsmokers (p >0.05). Compared with nonsmokers, in the left hemisphere, smokers showed lower FA values of PI-NAc (t= 5.784, p < 0.001)(Bonferroni corrected) and AI-NAc (t= 3.075,p= 0.003). In the right hemisphere, smokers showed increased FA values of AI-mOFC (t = -4.909, p < 0.001) , PI-mOFC (t = -4.727, p < 0.001) (Bonferroni corrected) and PI-NAc (t= -2.953,p= 0.004); decreased AD values of AI-mOFC (t = 2.100, p = 0.039) , PI-mOFC (t = 2.293, p =0.024) and PI-NAc (t = 2.435, p =0.017); decreased RD values of AI-mOFC (t = 3.071, p = 0.003) , PI-mOFC (t = 3.221, p =0.002) , and PI-NAc (t = 2.743, p =0.007); as well as decreased MD values of AI-mOFC (t = 2.854, p = 0.005) , PI-mOFC (t = 3.048, p =0.003),and PI-NAc (t = 2.908, p =0.005) (Fig. 2).Discussion and Conclusion
This study combined cortical and subcortical parcellation procedures with probabilistic tractography to examine white-matter fiber connections in reward pathways between insula, NAc and OFC in smokers. We identified reliable differences in the diffusion measurements of insula-NAc and insula-OFC tracts between smokers and nonsmokers. We observed lower FA of the left AI-NAc and PI-NAc tracts in smokers, suggesting decreased white-matter integrity of these insula tracts. Meanwhile, we observed higer FA, and lower AD, RD and MD in the right AI-mOFC, PI-mOFC, and PI-NAc tracts in smokers, suggesting excessive myelination and axonal loss of these insula tracts. These altered insula microstructural connectivity could interfere with the normal neural circuitry of reward processing. These results clarify the precise roles of insula white-matter microstructures in nicotine addiction, which may provide new insights into the underlying neurobiology of nicotine addiction.Acknowledgements
CW was supported by zhejiang provincial natural science foundation (LQ18H180001) and Medical and Health Scientific Research Fund Project of Zhejiang Province (2017KY080). MZ was supported by the Natural Science Foundation of China Surface Project (81171310).References
1. Chen Z, Peto R, Zhou M, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 2015;386: 1447-1456.
2. Volkow ND, Wang GJ, Fowler JS, et al. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011; 108: 15037-15042.
3. Engelmann JM, Versace F, Robinson JD, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012; 60: 252-262.
4. Shott ME, Pryor TL, Yang TT, Frank GK. Greater Insula White Matter Fiber Connectivity in Women Recovered from Anorexia Nervosa. Neuropsychopharmacology. 2016; 41: 498-507.
5. Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007; 315: 531-534.
6. Bi Y, Yuan K, Guan Y, et al. Altered resting state functional connectivity of anterior insula in young smokers. Brain Imaging Behav. 2017; 11: 155-165.
7. Stoeckel LE, Chai XJ, Zhang J, et al. Lower gray matter density and functional connectivity in the anterior insula in smokers compared with never smokers. Addict Biol. 2016; 21: 972-981.
Figures