4523
Microstructural and structural connectivity alterations in dexmedetomidine-induced loss of consciousness1Department of Neuroscience and Biomedical Engineering, Aalto University School of Science, Espoo, Finland, 2Turku Brain and Mind Center, University of Turku, Turku, Finland, 3Turku PET Centre, University of Turku and the Hospital District of Southwest Finland, Turku, Finland, 4Department of Intensive Care, Tampere University Hospital, Tampere, Finland, 5Division of Perioperative Services, Intensive Care and Pain Medicine, Turku University Hospital, University of Turku, Turku, Finland, 6Department of Psychology and Speech-Language Pathology, University of Turku, Turku, Finland, 7Department of Cognitive Neuroscience and Philosophy, School of Bioscience, University of Skövde, Skövde, Sweden, 8Department of Pharmacology, Drug Development and Therapeutics, University of Turku, Turku, Finland
Synopsis
We used diffusion MRI to investigate brain microstructure and structural connectivity in 10 healthy subjects before and during dexmedetomidine-induced loss of consciousness. We found rapid local changes both in the microstructural properties and in the structural brain connectivity networks, most prominently in the left angular gyrus and its connections indicating possible involvement of the area in consciousness. Moreover, our results indicate that conventional high b-value diffusion MRI acquisitions, in addition to sequences specifically designed to capture functional changes, are sensitive to at least major brain state changes.
Introduction
Diffusion MRI can be used to investigate brain microstructure and structural connectivity1. Modern acquisitions for the estimation of connectivity typically involve high diffusion-weighting (b>2500 s/mm2), while sequences designed to be sensitive to brain function, such as intra-voxel incoherent motion (IVIM), typically involve low diffusion-weighting (in IVIM, typically b=50-250 s/mm2). In this study, we used high diffusion-weighting to investigate whether rapid intra-subject microstructural and connectivity alterations could be observed during dexmedetomidine-induced loss of consciousness (LOC).Methods
We acquired diffusion-weighted (DW) and T1-weighted MRI data from 10 healthy subjects before and during dexmedetomidine-induced LOC. Dexmedetomidine was administered intravenously using target-controlled infusion until loss of responsiveness to verbal commands. Then, the drug concentration was increased by 50% to induce presumable LOC. Diffusion MRI data were acquired in 64 gradient orientations using a diffusion-weighting of b=3000 s/mm2 and three b=0 s/mm2 images. In addition, three b=0 s/mm2 images were acquired with reverse phase-encoding. The voxel size was 2 mm × 2 mm × 2 mm.The DW data were corrected for bias field2, subject motion3, eddy current induced4, and echo-planar imaging distortions5, and the structural data were aligned to the DW images with rigid body registration6. Cortical parcellation of the T1-weighted images was performed in FreeSurfer7 by using the Destrieux atlas8 combined with the subcortical gray matter structures segmented with FSL's9 FIRST10, resulting in 164 gray matter regions.
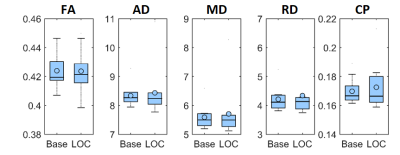
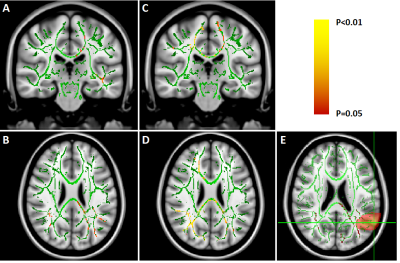
Then, global microstructural brain properties were calculated by performing constrained spherical deconvolution (CSD)-based11-12 whole-brain tractography13. We investigated traditional diffusion tensor-based microstructural metrics14, such as fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), and coefficient of planarity (CP)15. Local microstructural alterations along the white matter tract skeleton were investigated with tract-based spatial statistics (TBSS)16 and controlled for the family-wise error (FWE) rate using a significance threshold of α=0.05 with threshold-free cluster enhancement17.
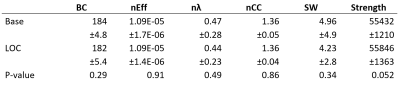
Structural brain connectivity networks18 were reconstructed by combining the parcellation8,10 with the streamlines reconstructed with CSD-based tractography12-13, resulting in a connectivity matrix of 164 × 164. Anatomically constrained tractography19 and spherical deconvolution informed filtering of tractograms (SIFT)20 were used to improve the anatomical correspondence of the reconstructed streamlines, resulting in 10 million streamlines. The number of streamlines connecting a pair of regions was used as the connection weight18. Global (betweenness centrality21, normalized global efficiency22, normalized characteristic path length23, normalized clustering coefficient24-25, small-worldness23, and strength) and local (betweenness centrality21 and local efficiency22) graph theoretical properties were investigated26-27. Normalization was performed by comparing the networks to 100 randomized networks with equal weight, degree, and strength distributions28. Statistical analyses were performed with a paired t-test and a corrected for FWE using a significance threshold of α=0.05.
Results
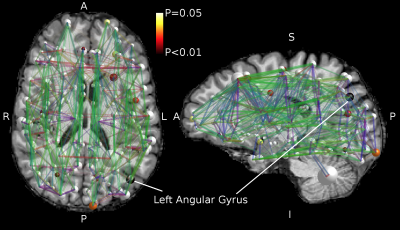
We found no global microstructural changes, as shown in Figure 1. However, significant local microstructural changes were observed with TBSS. MD was decreased in LOC on the left hemisphere, most prominently in the inferior fronto-occipital and inferior longitudinal fasciculi close to the left inferior parietal lobe (P<0.05). AD was decreased more widely, for example in the corpus callosum and more significantly on the right side (P<0.05). The local microstructural differences in MD and AD are shown in Figure 2. In addition, we observed a decrease in FA in the left anterior thalamic radiation and the fornix and an increase in RD in the sagittal stratum.In the structural brain connectivity networks, we found no global changes in the graph theoretical properties, as shown in Table 1. However, local betweenness centrality was significantly decreased (P=0.00027) in the left angular gyrus of the inferior parietal lobe (Figure 3). There were no other significant node-level findings after FWE-correction.
Discussion
We found no global microstructural or connectivity changes related to LOC. However, we observed significant local microstructural changes in MD and AD most prominently in the inferior parietal lobe, and significantly decreased node-level centrality in the left angular gyrus of the inferior parietal lobe in the structural brain networks.The overlap of the findings indicates that the inferior parietal lobe, more specifically the angular gyrus, may be associated with consciousness. Although the connectivity alteration was only observed unilaterally, the microstructural alterations were bilateral, and more pronounced for AD in the right hemisphere. The right angular gyrus has previously been reported in positron emission tomography studies of consciousness29-30. In addition, our results indicate that conventional diffusion MRI acquisitions are sensitive to major brain state changes, which may cause local alterations both in the observed brain microstructure and connectivity.
In the future, we plan to analyze more specific metrics such as fiber density and cross section31 and investigate their relationship to both structural and functional brain connectivity.
Conclusion
We found rapid local abnormalities related to dexmedetomidine-induced LOC in structural brain connectivity of the left angular gyrus in the inferior parietal lobe, and in the local brain microstructure of the corpus callosum and near the inferior parietal lobe.Acknowledgements
This work was supported by the Emil Aaltonen Foundation, the Finnish Cultural Foundation, the Academy of Finland (grants #266467 and #266434), and the Jane and Aatos Erkko Foundation.References
1. Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magnet Reson Med. 2011;65(6):1532-1556.
2. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imag. 2001;20(1):45-57.
3. Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magnet Reson Med. 2009;61(6):1336-1349.
4. Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063-1078.
5. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20(2):870-888.
6. Ashburner J, Friston K. Rigid body registration, in: Human Brain Function. 2nd ed. London: Academic Press, 2003;635-655.
7. Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11-22.
8. Destrieux C, Fischl B, Dale A, et al. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1-15.
9. Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. NeuroImage. 2012;62(2):782-790.
10. Patenaude B, Smith SM, Kennedy DN, et al. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56(3):907-922.
11. Tournier JD, Calamante F, Gadian DG, et al. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage. 2004;23(3):1176-1185.
12. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. NeuroImage. 2007;35(4):1459-1472.
13. Tournier JD, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fiber regions. Int J Imag Syst Tech. 2012;22(1):53-66.
14. Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magnet Reson Series B. 1994;103(3):247-254.
15. Westin CF, Maier SE, Mamata H, et al. Processing and visualization for diffusion tensor MRI. Med Imag Anal. 2002;6(2):93-108.
16. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487-1505.
17. Smith S, Nichols T. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83-98.
18. Roine T, Jeurissen B, Perrone D, et al. Reproducibility and intercorrelation of graph theoretical measures in structural brain connectivity networks. Med Imag Anal. 2019;52:56-67.
19. Smith RE, Tournier JD, Calamante F, et al. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage. 2012;62(3):1924-1938.
20. Smith RE, Tournier JD, Calamante F, et al. SIFT: spherical-deconvolution informed filtering of tractograms. NeuroImage. 2013;67:298-312.
21. Brandes U. A faster algorithm for betweenness centrality. J Math Sociol. 2001;25:163–177.
22. Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701.
23. Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature. 1998;393:440-2.
24. Onnela JP, Saramäki J, Kertész J, et al. Intensity and coherence of motifs in weighted complex networks. Phys Rev E - Stat Nonlinear Soft Matter Phys. 2005;71(6):065103.
25. Saramäki J, Kivelä M, Onnela JP, et al. Generalizations of the clustering coefficient to weighted complex networks. Phys Rev E - Stat Nonlinear Soft Matter Phys. 2007;75(2):027105.
26. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186.
27. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52(3):1059-1069.
28. Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. NeuroImage. 2011;56:2068–2079.
29. Laureys S, Goldman S, Phillips C, et al. Impaired Effective Cortical Connectivity in Vegetative State: Preliminary Investigation Using PET. NeuroImage. 1999;9(4):377-382.
30. Fiset P, Paus T, Daloze T, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci. 1999;19(13):5506-5513.
31. Raffelt DA, Tournier JD, Smith RE, et al. Investigating white matter fibre density and morphology using fixel-based analysis. NeuroImage. 2017;144:58-73.
Figures