4521
Increased non-Gaussian subdiffusion in white matter is associated with increased longitudinal blood pressure exposure in adults at midlife1Department of Neurology, Northwestern University, Chicago, IL, United States, 2Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, IL, United States, 3Department of Neurology, Chicago, IL, United States, 4Department of Radiology, Northwestern University, Chicago, IL, United States, 5Department of Psychiatry and Behavioral Sciences, Northwestern University, Chicago, IL, United States, 6Department of Preventative Medicine, Northwestern University, Chicago, IL, United States
Synopsis
The increased presence of non-Gaussian subdiffusive dynamics, possibly reflecting the presence of increased neuronal and glial microstructural heterogeneity, is sensitive increased vascular risk exposure, which was not observed with traditional DTI metrics such as FA.
Introduction
There has been growing interest to investigate normal-appearing white matter (NAWM) microstructural integrity via diffusion tensor imaging (DTI) in various neuropathophysiologies1, that is not classified as lesioned tissue via white matter hyperintensities FLAIR imaging2. However, DTI is just one modeling technique to interpret diffusion-weighted data and is only valid for a limited MRI data acquisition scheme3,4. To more completely describe diffusion-weighted signal, there have been attempts to implement a diffusion model that estimates kurtosis as a measure of non-linear dynamics5,6, but even this method has limitations on data acquisition requirements7. There has been recent work to model these non-linear diffusion dynamics as anomalous subdiffusion as a way to identify tissue microstructural complexity that has been shown to be sensitive to both axonal and glial cell morphology8. In this study, we perform a region of interest DTI and complexity analysis of NAWM to determine the relationship between diffusivity metrics and increased risk for blood pressure exposure for a cohort of participants in midlife that have been enrolled thus far in an ongoing 30 year study to identify Coronary Artery Risk Development in Young Adults (CARDIA)9.Methods
The study has been approved by the institutional review board of Northwestern University. After the Year 30 visit in the CARDIA study, separate written consent was obtained where upon 77 participants were scanned (Table 1). Diffusion-weighted images were acquired with the following parameters on a 3 Tesla Siemens Prisma scanner: TE=76.8ms, TR=3000ms, flip angle=90°, matrix=150x150, FOV=225x225mm2, resolution=1.5x1.5x1.5mm3, slices=90, b0 averages=9, diffusion gradient directions=90, diffusion-weighting = 1000, 2000, 3000s/mm2, multiband acceleration factor=4. FLAIR images were acquired with the following: TE=289ms, TR=6000ms, TI=2200ms, flip angle=120°, matrix=256x256, FOV= 256x256mm2, resolution = 1x1x1mm3. The raw diffusion data were denoised, brain extracted, linear registered, affine registered to correct for eddy current distortions, and diffusion gradient direction were corrected based on motion parameters. The diffusion tensor parameters were be calculated from the preprocessed b=0 and b=1000s/mm2 diffusion data to generate fractional anisotropy (FA) maps. The b=0, 1000, 2000, 3000s/mm2 diffusion data were used to estimate the power law subdiffusion index, α, in the following equation, S(b)/S(0)=Eα(-bD), where Eα is the single parameter Mittag-Leffler function, which describes subdiffusion and power law dynamics7,10. Overall, α serves as a heterogeneity index (0<α≤1) to determine the deviation from simple homogeneous Gaussian diffusion (α~1), and the smaller the value of α, the more heterogeneous the diffusion, indicative of power law subdiffusive behavior and an increasingly complex diffusion environment. Tract-based Spatial Statistics (TBSS)11 were performed using the FA and α images for statistical analyses of the cohort white matter characteristics in MNI space.Results
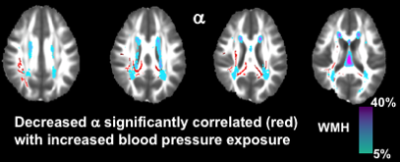
There were no significant associations between FA and longitudinal blood pressure exposure. There was a significant negative association between α and longitudinal blood pressure exposure (r=-0.362, p=0.032), when adjusting for all other demographic and clinical risk factors as covariates (Table 1). Shown in Figure 1, this relationship indicates that decreased α, representing increased presence of non-Gaussian subdiffusion dynamics in NAWM, was correlated with increased risk of blood pressure exposure.Discussion
While it could seem counterintuitive to observe an increased presence of non-Gaussian subdiffusive dynamics in NAWM which is associated with increased vascular exposure, this phonemenon is not without precedent. In a recent rodent study, α was estimated in both wild type mice and those mice which were genetically altered to develop symptoms of Huntington’s disease12. The results of the rodent study demonstrated decreased α in the corpus callosum for the Huntington’s mice in comparison to the control mice. Along with these diffusion metrics, the optical images demonstrated evidence of not only dysmyelinated axons, but also an increase in density count of glial cells for the Huntington's mice12. Therefore, the multifaceted combination of axonal degeneration and proliferation of glial cells as an inflammatory immune response of neural repair is possibly represented by increased presence non-Gaussian subdiffusive dynamics as the heterogeneity of cell type was increased for the Huntington’s mice. In the context of the results of the present study and previous work, non-Gaussian subdiffusive dynamics in NAWM which is associated with increased vascular exposure during midlife could possibly be explained by a combination of axonal disruption, increased inflammatory mechanisms, and increased glial proliferation. The contribution of glial cells to the MRI signal should not be ignored, as it has previously been estimated to comprise about 40% of a purely white matter voxel13. Considering all possible relevant covariates for correction there appears to be altered brain microstructure in NAWM for those with increased vascular risk that could be indicative of physiological response differences at midlife. It should be noted that there is a direct mathematical conversion of the non-Gaussian subdiffusion parameter7, α, estimated in this study and kurtosis, K, in which case would indicate that increased kurtosis is associated with increased longitudinal blood pressure exposure at midlife.Conclusion
Estimation of non-Gaussian subdiffusive dynamics is made possible for various applications of neuropathophysiolgy with the acquisition of a mult-shell diffusion-weighted imaging protocol. In the current study, the increased presence of non-Gaussian subdiffusive dynamics, possibly reflecting the presence of increased neuronal and glial microstructural heterogeneity, is sensitive increased vascular risk exposure, which was not observed with traditional DTI metrics such as FA.Acknowledgements
This study was supported by National Institute of Neurological Disorders and Stroke (NINDS; R01-NS085002). The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). CARDIA was also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005).References
1. de Groot, M., Verhaaren, B.F., de Boer, R., Klein, S., Hofman, A., van der Lugt, A., Ikram, M.A., Niessen, W.J., Vernooij, M.W., 2013. Changes in normal-appearing white matter precede development of white matter lesions. Stroke 44, 1037-1042.
2. Henninger, N., Lin, E., Haussen, D.C., Lehman, L.L., Takhtani, D., Selim, M., Moonis, M., 2013. Leukoaraiosis and sex predict the hyperacute ischemic core volume. Stroke 44, 61-67.
3. Ingo, C., Magin, R.L., Colon-Perez, L., Triplett, W., Mareci, T.H., 2014. On random walks and entropy in diffusion-weighted magnetic resonance imaging studies of neural tissue. Magn Reson Med 71, 617-627.
4. Magin, R.L., Abdullah, O., Baleanu, D., Zhou, X.J., 2008. Anomalous diffusion expressed through fractional order differential operators in the Bloch-Torrey equation. J Magn Reson 190, 255-270.
5. Jelescu, I.O., Zurek, M., Winters, K.V., Veraart, J., Rajaratnam, A., Kim, N.S., Babb, J.S., Shepherd, T.M., Novikov, D.S., Kim, S.G., Fieremans, E., 2016. In vivo quantification of demyelination and recovery using compartment-specific diffusion MRI metrics validated by electron microscopy. NeuroImage 132, 104-114.
6. Jensen, J.H., Helpern, J.A., Ramani, A., Lu, H., Kaczynski, K., 2005. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine 53, 1432-1440.
7. Ingo, C., Sui, Y., Chen, Y., Parrish, T., Webb, A., Ronen, I., 2015. Parsimonious Continuous Time Random Walk Models and Kurtosis for Diffusion in Magnetic Resonance of Biological Tissue. Frontiers in Physics 3.
8. Ingo, C., Brink, W., Ercan, E., Webb, A.G., Ronen, I., 2018. Studying neurons and glia non-invasively via anomalous subdiffusion of intracellular metabolites. Brain Struct Funct 223, 3841-3854.
9. Hughes, G.H., Cutter, G., Donahue, R., Friedman, G.D., Hulley, S., Hunkeler, E., Jacobs, D.R., Jr., Liu, K., Orden, S., Pirie, P., et al., 1987. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials 8, 68S-73S.
10.
Metzler, R., Klafter, J., 2000.
The random walk's guide to anomalous diffusion: a fractional dynamics approach.
Physics Reports 339, 1-77.
11.
Smith, S.M., Jenkinson, M.,
Johansen-Berg, H., Rueckert, D., Nichols, T.E., Mackay, C.E., Watkins, K.E.,
Ciccarelli, O., Cader, M.Z., Matthews, P.M., Behrens, T.E., 2006. Tract-based
spatial statistics: voxelwise analysis of multi-subject diffusion data.
NeuroImage 31, 1487-1505.
12. Gatto, R.G., Ye, A.Q., Colon-Perez, L., Mareci, T.H., Lysakowski, A., Price, S.D., Brady, S.T., Karaman, M., Morfini, G., Magin, R.L., 2019. Detection of axonal degeneration in a mouse model of Huntington's disease: comparison between diffusion tensor imaging and anomalous diffusion metrics. Magn Reson Mater Phy 32, 461-471.
13.
Walhovd, K.B., Johansen-Berg,
H., Karadottir, R.T., 2014. Unraveling the secrets of white matter--bridging
the gap between cellular, animal and human imaging studies. Neuroscience 276,
2-13.
Figures