4516
Application Study of DWI Radiomics Features with Transurethral Resection on Assessing the Muscular Infiltrating of Bladder Carcinoma1Radiology, Renji Hospital,Shanghai Jiaotong University School of Medicine, Shanghai, China, 2United Imaing Healthcare, Shanghai, China
Synopsis
To investigate the value of radiomics features from diffusion-weighted imaging (DWI) in differentiating muscle-invasive bladder cancer (MIBC) from non-muscle-invasive bladder cancer (NMIBC). Combining DWI radiomics features with TUR could improve the sensitivity and accuracy in discriminating the presence of muscle invasion in bladder cancer for clinical practice. Multi-center, prospective studies are needed to confirm our results.
INTRODUCTION
To investigate the value of radiomics features from diffusion-weighted imaging (DWI) in differentiating muscle-invasive bladder cancer (MIBC) from non-muscle-invasive bladder cancer (NMIBC).METHODS
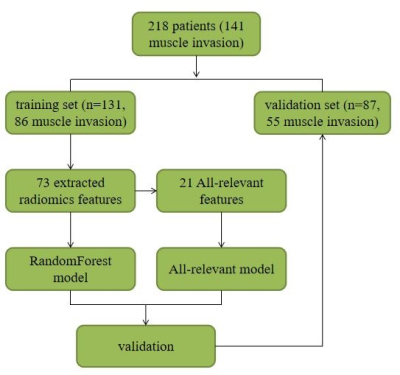
This retrospective study included 218 pathologically-confirmed bladder cancer patients (training set: 131 patients, 86 MIBC; validation set: 87 patients, 55 MIBC) who underwent DWI before biopsy through transurethral resection (TUR) between July 2014 and December 2018. Radiomics models based on DWI for discriminating state of muscle-invasive were built using random forest (RF) and all-relevant (AR) methods on the training set and were tested on validation set. Combination models based on TUR data were also built. Discrimination performances were evaluated with the area under the receiver operating characteristic (ROC) curve (AUC), accuracy, sensitivity, specificity, F1 and F2 scores. Qualitative MRI evaluation based on morphology was performed for comparison.RESULTS
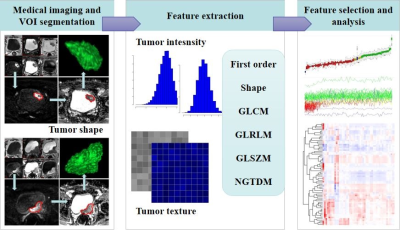
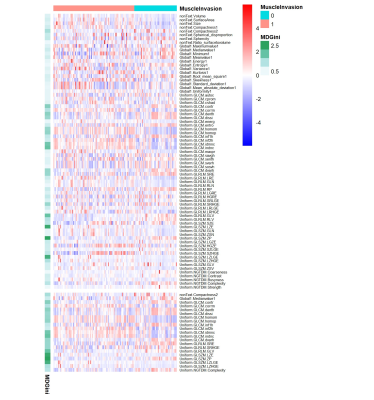
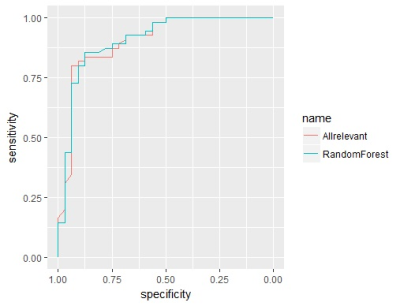
Patient population 245 patients were included. After excluding 37 patients in whom radiomics features could not be extracted due to the small volume of lesions or the limited visibility of images, 218 (169 males; mean age, 66.1 yrs [range, 37-93]; 141 muscle-invasive tumors) were left for further analyses. In this patient group, TUR only confirmed 87 muscle-invasive tumors, and 38.3% (54/141) of RC-confirmed muscle-invasive tumors were misdiagnosed as non-muscle-invasive tumors at TUR. Patients were randomly divided into training set (131 patients; 104 males; mean age, 65.8 yrs [range, 38-86]; 86 muscle-invasive tumors) and validation set (87 patients; 65 males; mean age, 66.5 yrs [range, 37-93]; 55 muscle-invasive tumors)(Fig 1). No significant difference was observed in age (p=0.696, wilcoxon rank sum test), gender (p=0.519, chi-squared test) or muscle invasion (p=0.824, chi-squared test) between the two sets . 73 features with ICC of more than 0.85 were extracted by different methods, including first order, shape, GLCM, GLRLM, GLSZM, and NGTDM features. After Boruta selection, 21 all-relevant features were obtained (Fig 2-3). Internal validation showed no significant difference in AUC (0.907 vs 0.904, p=0.673, Delong’s test), ACC (0.839 vs 0.816, p=0.480, McNemar’s test), SEN (0.873 vs 0.855, p=1.000), or SPE (0.781 vs 0.750, p=1.000) between RandomForest model and All-relevant model for discriminating muscle-invasive BC (Fig 4). RandomForest model was more sensitive than TUR (0.873 vs 0.655, p=0.019, McNemar’s test), and MRI (0.873 vs 0.764, p=0.181) for discriminating MIBC, but the difference did not reach statistical significance. When combining the RandomForest model with TUR, the sensitivity increased to 0.964, significantly higher than TUR (0.655, p<0.001), MRI (0.764, p=0.006), and the combination of TUR and MRI (0.836, p=0.046). Notably, the combination model (RandomForest model and TUR) had the highest accuracy of 0.897 and F2 score of 0.946 for discriminating MIBC.DISCUSSION
In this study, 38.3% (54/141) of RC-confirmed muscle-invasive tumors were misdiagnosed as non-muscle-invasive tumors at TUR, which is consistent with previous reports [1,2-4]. Many reasons account for the poor sensitivity of TUR for discriminating muscle-invasive tumors, such as sampling error due to incompleteness of TUR, delay in the interval from TUR to RC, and poor sensitivity of preoperative staging tools [1,2]. Besides, qualitative MRI evaluation only showed a good inter-observer repeatability (Kappa value = 0.605) and a poor sensitivity comparable to that of TUR (0.764 vs 0.873, p=0.181). Our radiomics model exhibited favorable discrimination performance in internal validation, with an AUC of 0.907 on the test set. The obvious advantage of TUR is its specificity of 100%, as muscle invasion is confirmed once observed at TUR specimen without considering the pathological result at RC. But for detecting highly malignant muscle-invasive BC, what physicians most importantly need is a more sensitive staging tool with a false negative rate as low as possible altogether with a relatively high positive predictive value (PPV). Recall (sensitivity) is more important than precision (PPV). Considering that F1 score is the harmonic average of the precision and recall, and that F2 score weighs recall higher than precision by placing more emphasis on false negatives, our radiomics model and combination model showed improved performance for discriminating muscle-invasive BC compared with TUR and qualitative MRI evaluation as seen on Table 3. Another major finding of this study was that a small subset of all-relevant radiomics features selected by Boruta exhibited an equivalent performance compared to that of all the extracted features, although the classification performance using the selected optimal feature subset outperformed that using the candidate feature set in a previous report [5]. Our finding suggested that radiomics data contained redundant or irrelevant features and that feature selection should be performed in building radiomics models.CONCLUSION
Combining DWI radiomics features with TUR could improve the sensitivity and accuracy in discriminating the presence of muscle invasion in bladder cancer for clinical practice. Multi-center, prospective studies are needed to confirm our results.Acknowledgements
The authors thank their colleagues of the department of radiology of their institute.References
[1] Babjuk M, Bohle A, Burger M et al (2017) EAU guidelines on non-muscle invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 71:447-61
[2] Karakiewicz PI, Shariat SF, Palapattu GS et al (2006) Precystectomy nomogram for prediction of advanced bladder cancer stage. Eur Urol 50:1254-1260
[3] Shariat SF, Palapattu GS, Karakiewicz PI et al (2007) Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol 51:137-149;discussion 49-51
[4] Svatek RS, Shariat SF, Novara G et al (2011) Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int 107:898-904
[5] Zhang X, Xu X, Tian Q et al (2017) Radiomics assessment of bladder cancer grade using texture features from diffusion-weighted imaging. J Magn Reson Imaging 6:1281-1288
Figures