4513
Rigorous Prospective Reduction of Inter-scanner Variance of Diffusion Imaging: Initial Experience1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Biomedical Informatics, University of Pittsburgh, Pittsburgh, PA, United States, 3Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 4Radiology, University of Michigan, Ann Arbor, MI, United States, 5Radiology, Harvard Medical School, Boston, MA, United States, 6Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 7Radiology, Emory University, Atlanta, GA, United States, 8Psychology, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
Our study shows for the first time that synthetic phantoms that simulate fiber anatomical characteristics can provide in vitro corrections factors for reducing in-vivo inter-scanner variance specific to discrete segments of cortical association fiber tracts. The overall goal of our evolving rigorous harmonization approach is to reduce inter-scanner variance that can confound biological variance derived from multi-center discovery and neuroprotection clinical trials in the developing human brain.
Introduction
Diffusion tensor imaging (DTI) is an important biomarker for multi-center discovery and neuroprotection clinical trial for the developing brain. DTI is sensitive to the scanner hardware specifications, sequence parameters, and field inhomogeneities and has relatively higher inter-scanner variance which can confound biological variance1. Current harmonization techniques like COMBAT can leverage normative cohorts to retrospectively reduced inters-canner variance, but may be limited in the evaluation of clinical populations that are being testing in multi-centered studies2. Here, for the first time, we aimed to develop more rigorous prospectively harmonization tool to incorporate in vitro synthetic phantom3,4 measurements of (1) linear packing fiber density (FA/fiber density correlation) and (2) crossing fiber density (FA/crossing angle) utilizing similar DTI and HARDI acquisition protocols and along tract post-processing5 across synthetic phantoms, human phantom (n=5), and human clinical subjects 10-12 yrs (Hypoplastic Left Heart Syndrome and age-matched healthy controls). We tested the feasibility of deriving an in vitro correction factor to reduce in vivo inter-scanner variance of both linear and crossing fiber segments of three types of tracts: (1) Cortical Spinal Tract; (2) Interhemispheric -Callosal Tract; (4) Intrahemispheric Tract (superior longitudinal fasiculus).Methods
The synthetic and five human phantoms were scanned at twelve participating sites at 3T at three separate sessions for a total of 216 times. The scanners include three Siemens Skyra (S Skyra), five Siemens Prisma (S Prisma), one Siemens Trio, two Philips Ingenia (P Ingenia), and one Philips Achieva (P Achieva). Diffusion imaging was acquired using both a 42-direction DTI and a 256-direction diffusion set for high angular definition diffusion imaging (HARDI) scheme. For the DTI the following parameters were used across all platforms: FOV = 256mm, voxel size = 2.0mm (isotropic), TE/TR=92ms/12600 ms, B = 1000s/mm2.As shown in Figure 1, the synthetic phantom contains blocks with unidirectional longitudinal fibers at different densities – 12.5%, 25%, 50%, and 100% density by volume. Average fractional anisotropy measurements over ROIs were generated for the density blocks in DSI studio6. An estimate of the linear relationship between FA and reference density was determined using regression analysis. Our modeling for FA and crossing fiber density utilizing HARDI is in progress. From this initial analysis we generated a scanner-specific reference measurement correction factor which is then applied to a second synthetic scan from the same scanner.
Each DTI scan of each human phantom are registered to other scans of the same individual using FSL FLIRT7 to incorporate all the brain images into a common person-specific space. Tractography was performed on DSI studio using a standardized scheme employing a uniform set of ROIs and ROAs to generate, for the initial study, 11 white matter tracts: Genu, CC Body, Splenium, left and right CST, left and right SLF, left and right ILF, and left and right FoF.
Results
Our preliminary results from the currently ongoing analysis examines scans at seven sites. Figure 2 shows the correction calculations using FA measurements from DTI imaging of the synthetic phantom unidirectional fiber blocks. The first set of scans (Figure 2A) were used to determine the scanner specific correction factors which were then applied to the second set of scans.For all scanners, the unidirectional fibers showed a linear relationship between fiber density and corresponding FA measurements, with FA increasing at higher fiber density. After the correction (Figure 2C), a reduction in cross scanner variability is observed (decreased in mean squared error) in all scanners. We also observed that the initial processing of 12.5% density blocks have low SNR and were excluded from this preliminary analysis.
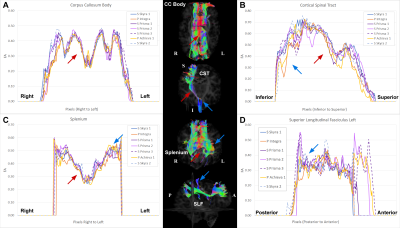
As shown in Figure 3 along-tract analysis where average FA (or other diffusion metric) for cross sectional area of fiber bundle are calculated along the longitudinal axis of each tract. The graphs show the along-tract FA measurements of CC Body, Splenium, Right CST, and Left SLF from one human phantom scanned at multiple sites. This initial analysis demonstrated, present to varying degrees in all the tracts, regions that are more sensitive to scanner variability (blue arrows) as well as regions that are reproducibly and reliably measured (red arrows) regardless of MRI used. Both CC Body and Splenium showed lower variance and higher measurement reproducibility (or convergence) around the midline of the tract, while wider variability is seen on the more lateral regions, especially in the Splenium. In the CST, highly variable FA measurements across scanners is seen near the inferior part of the tract, perhaps due to the abundance of decussating fibers in that region. A region with minimized variability is seen superiorly, corresponding to the internal capsule. The SLF exhibit the highest variability among the presented tracts. This is perhaps due to its more dispersed nature, which in turn render this tract more sensitive to the intrinsic scanner factors.
Discussion
Our study shows for the first time that synthetic phantoms that simulate fiber anatomical characteristics can provide in vitro corrections factors for reducing in-vivo inter-scanner variance specific to discrete segments of cortical association fiber tracts. The overall goal of our evolving rigorous harmonization approach is to reduce inter-scanner variance that can confound biological variance derived from multi-center discovery and neuroprotection clinical trials in the developing human brain.Acknowledgements
Christine Johnson, Nancy Beluk, Julia WallaceReferences
1. Mirzaalian, Hengameh, Lipeng Ning, Peter Savadjiev, Ofer Pasternak, Sylvain Bouix, O. Michailovich, G. Grant et al. "Inter-site and inter-scanner diffusion MRI data harmonization." NeuroImage 135 (2016): 311-323.
2. Fortin, Jean-Philippe, Drew Parker, Birkan Tunç, Takanori Watanabe, Mark A. Elliott, Kosha Ruparel, David R. Roalf et al. "Harmonization of multi-site diffusion tensor imaging data." Neuroimage 161 (2017): 149-170.
3. Guise, Catarina, Margarida M. Fernandes, Joao M. Nobrega, Sudhir Pathak, Walter Schneider, and Raul Fangueiro. "Hollow polypropylene yarns as a biomimetic brain phantom for the validation of high-definition fiber tractography imaging." ACS applied materials & interfaces 8, no. 44 (2016): 29960-29967.
4. Wilde, Elisabeth A., James M. Provenzale, Brian A. Taylor, Michael Boss, Anthony Zuccolotto, Rebecca Hachey, Sudhir Pathak, David F. Tate, Tracy J. Abildskov, and Walter Schneider. "Assessment of quantitative magnetic resonance imaging metrics in the brain through the use of a novel phantom." Brain injury 32, no. 10 (2018): 1265-1275.
5. Ceschin, Rafael, Vince K. Lee, Vince Schmithorst, and Ashok Panigrahy. "Regional vulnerability of longitudinal cortical association connectivity: Associated with structural network topology alterations in preterm children with cerebral palsy." NeuroImage: Clinical 9 (2015): 322-337.
6. Yeh, Fang-Cheng, Timothy D. Verstynen, Yibao Wang, Juan C. Fernández-Miranda, and Wen-Yih Isaac Tseng. "Deterministic diffusion fiber tracking improved by quantitative anisotropy." PloS one 8, no. 11 (2013): e80713.
7. M. Jenkinson and S.M. Smith. A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2):143-156, 2001.
Figures