4512
Impact of processing pipelines on biological findings of large scale multicenter DTI studies1National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD, United States, 2Henry M. Jackson Foundation for the Advancement of Military Medicine, Rockville, MD, United States
Synopsis
Different post-processing pipelines might influence the research or even clinical conclusions obtained from diffusion MRI. Our study investigated the impact of alternative pre-processing techniques and spatial normalization approaches on the outcome of a large multicenter study in schizophrenia that was originally analyzed using the ENIGMA pipeline. Our main finding is the discovery of profound effects of the spatial normalization approach used, on both biological conclusions and inter-site harmonization of the results.
Introduction
Diffusion magnetic resonance imaging (MRI) including diffusion tensor imaging (DTI) has been widely used for investigating brain development, aging, psychiatric and neurological disorders. However, diffusion MRI data suffer from a number of artifacts, such as subject movement, eddy current distortion, and susceptibility induced EPI distortion.1 There is a general consensus that appropriate processing methods are crucial for obtaining accurate diffusion MRI results, however, in large studies, a single pipeline is typically employed and results from alternative processing strategies are not investigated. The purpose of this study was to investigate the impact of two aspects: 1) the choice of processing algorithm for eddy current distortion correction and motion correction of the diffusion weighted images (DWIs) and 2) the choice of spatial normalization strategies to perform population analysis of fractional anisotropy (FA) results. As test dataset we used data from previously published large multicenter study in schizophrenia (SCZ) that were originally analyzed using the ENIGMA pipeline.Methods
The study included a total of 401 subjects (172 SCZ patients / 229 health controls [HCs]) from three sites. The algorithms tested for DWI preprocessing included: “FSL eddy_correct”, which was used by the authors of the original publication,2 “FSL eddy” ver. 6.0, and, TORTOISE ver. 3.1.3 (www.tortoisedti.org). The outcome metric we analyzed was the mean value of the whole brain “skeletonized” FA,3 which was the same outcome metric considered in the original publication.For spatial normalization, the original analysis used FA registration to the ENIGMA FA template. A full tensor template is not available for the ENIGMA template. We created study- specific diffusion tensor (DT) and FA templates using the DR-TAMAS software.4 DR-TAMAS performs tensor-based spatial normalization of DTs of all subjects included in the study producing an average DT template, from which a FA template is computed. We also used the JHU template which are available the full tensor and FA templates. In addition to the FA registration to the FA ENIGMA template, we added the following spatial normalization approaches: FA registration to the JHU FA template, FA registration to the study specific template, tensor-based registration to the JHU DT template, and tensor-based registration to the DT study-specific template.
As biological outcome metric we investigated the same metric analyzed in the original paper. The age of peak for the FA for all the various pipelines was estimated.
Results and Discussion
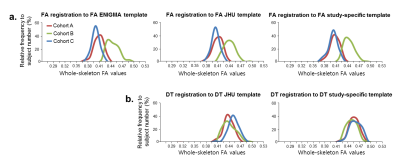
Fig. 1 shows that different eddy correction methods result in very different average FA values. The FA values obtained with eddy_correct processing were significantly lower than those obtained with eddy and TORTOISE. It should be noted that eddy_correct uses an affine model of eddy current distortion, which is insufficient to achieve full correction,5 so it is likely that the lower FA values obtained by eddy_correct are artifactual. However, to our knowledge, both eddy and TORTOISE use an appropriate model for eddy-current distortion correction but they still generate substantially different FA values.Fig. 2 shows the distribution of FA values for the control subjects at each site, with processing obtained from the different spatial normalization approaches we tested. The results processed using FA registration to the FA ENIGMA template show a significant lack of harmonization across sites. Inter-site harmonization is slightly improved using the FA JHU and FA study specific template. However, significant discrepancies between site B and the other two sites persist. Inter-site harmonization was dramatically improved by using DT registration, with the best agreement reached for the DT registration to DT study specific template.
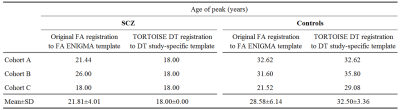
Table 1 shows the age of peak FA values computed in the original publication (eddy_correct, FA registration to FA ENIGMA template), and computed from data processed using TORTOISE and DT registration to the DT study-specific template. The original processing approach resulted in large variability of the results across sites for both patients and controls. The TORTOISE processing resulted in much lower inter-site variability. Moreover, the results obtained by the TORTOISE approach indicate much larger differences between patients and controls, and suggest that white matter degenerative changes in SCZ originate early in life.
Conclusion
We expected that different diffusion processing pipelines may produce slightly different results when applied to the same diffusion MRI data acquired in multi-center studies. Our main hypothesis was that inter-group variability would be affected. One of the most surprising findings of our investigation, however, is that diffusion MRI processing approaches may introduce significant bias in the results, creating an apparent lack of inter-site harmonization. We also discovered an important effect of the choice of method and template for spatial normalization. The biological conclusions that one can reach from the same data are strongly affected by the processing approach employed.Acknowledgements
No acknowledgement found.References
1. Karlsgodt KH. Diffusion Imaging of white matter in Schizophrenia: Progress and Future Directions. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(3):209-217.
2. Kochunov P, Ganjgahi H, Winkler A, et al. Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp. 2016;37(12):4673-4688.
3. Smith SM, Johansen-Berg H, Jenkinson M, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499-503.
4. Irfanoglu MO, Nayak A, Jenkins J, et al. DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. Neuroimage. 2016;132:439-454.
5. Rohde GK, Barnett AS, Basser PJ, et al. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn Reson Med. 2004;51(1):103-14.
Figures