4499
Sensitivity Gain from Multi-Echo Acquisitions in Ex-Vivo Diffusion Imaging: Numerical Simulations and Experimental Verification1Department of Neuropsychology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2NMR Unit, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany, 4Paul Flechsig Institute of Brain Research, University Leipzig, Leipzig, Germany
Synopsis
Ex-vivo dMRI acquisitions achieve very high resolutions from increased SNR at the cost of prolonged scan times. Due to highly-segmented acquisition strategies, the ex-vivo dMRI signal is often not fully decayed when signal encoding progresses. Using numerical simulations and real-dMRI data from a wild chimpanzee, we show that this circumstance can be effectively employed using an optimized noise informed combination of multi-echo acquisitions from segmented EPI trains. The additional sampling comes at a small price and leads to increased SNR and a general reduction of signal bias.
Introduction
Ex-vivo diffusion MRI (dMRI) enables structural data acquisitions with significantly higher anatomical detail than typical in-vivo acquisitions, resulting from extended scan times1,2. However, ex-vivo dMRI faces two main problems. Firstly, reduced diffusivity of formalin-fixed tissue requires stronger diffusion-weightings compared to in-vivo tissue, resulting in signal-loss from increased TEs. Second, susceptibility jumps arising from trapped air bubbles and container boundaries result in severe image-distortions. Segmented EPI (sEPI) acquisitions can counteract these problems by diminishing the effective echo durations, thereby reducing echo times and image distortions. SEPI acquisitions have extremely short readout-trains, allowing the addition of multiple gradient-echo (GRE) acquisitions with only negligible increases in acquisition time3. In this work, a Multi-Echo (ME) sEPI dMRI sequence was developed to achieve non-biased signal reconstructions with increased SNR for ex-vivo dMRI acquisitions. To achieve this goal, a T2* decay model and statistical noise modeling were combined to reconstruct high-SNR ME-dMRI data. The developed acquisition strategy was employed to acquire high-resolution dMRI data of an ex-vivo wild chimpanzee brain on a human-scale MRI system.Theory
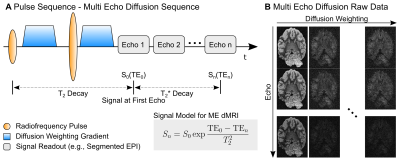
The ME-dMRI signal is described using a mixed decay model (Fig1). Here, the decay up to the first echo time (TE0) follows T2-relaxation, whereas the N later echoes follow T2*-relaxation. ME-MRI data are often combined using weighted summations3,4. We here employ the data from multiple echoes to estimate S0 using the ME-dMRI signal-model. The SNR gain factor of the fitting-approach is approximated by Eq1:$$G_{\text{SNR}} = \frac{N}{\exp{ \left( - \frac{\text{TE}_0}{T_2^*} \right)} } \sqrt{\sum_n^N{ \left( \exp{ \frac{\text{TE}_n}{T_2^*} } \right)^2} } $$
We evaluated two different fitting-strategies: Linear Least Squares (LLS) and Maximum Likelihood Estimation (MLE)5. Unlike LLS, MLE incorporates the underlying non-Gaussian noise distribution into the parameter estimation.
Methods
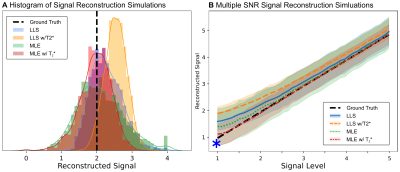
MR Acquisition: MRI data were acquired from the brain of a wild chimpanzee from Taï National Forest (Ivory Coast), deceased from a natural cause. The brain was extracted four hours after death, immersion-fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS), washed in PBS and immersed in perfluoropolyether for MR scanning. ME-dMRI data were acquired at a 3T MR system (Connectom, Siemens, Germany) with Gmax=300mT/m using a 32-channel phased-array head coil with the following imaging parameters: 0.8×0.8×0.8mm3, FoV=128×96mm2, TR=6105ms, TE=[45.0,50.9,56.8,62.7,68.6]ms, 40 segments, Adaptive-Combine Coil-Combination, no PF, no GRAPPA. Following a complete dummy-acquisition to mitigate short-term instabilities2, three repetitions of 60 diffusion-weighted volumes were acquired at b=5000s/mm2. Echo time-shifting was employed to minimize phase-discrepancies between segments6. A noise map was acquired using identical protocol-parameters without RF-excitation. To compute a quantitative T2* map, a ME-FLASH sequence with 21 echoes at matching resolution was acquired.Reconstruction Simulations: To evaluate ME-dMRI fitting-strategies, numerical simulations within the relevant parameter range were employed. Synthetic signal-decays, matching the ME-dMRI acquisition protocol, were generated for various S0 intensities and corrupted with "Rician" noise ($$$\sigma=1$$$, the data follows a Rician distribution). S0 was reconstructed using LLS and MLE, both with the additional incorporation of known T2* decay. For each signal-level, 1000 repetitions were computed.
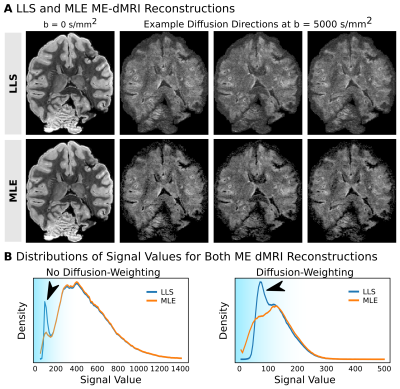
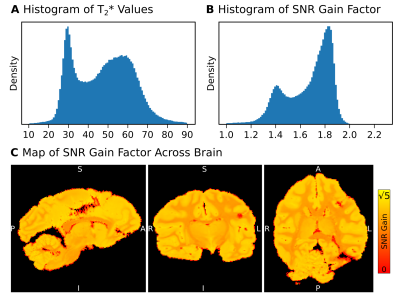
MR Reconstruction: ME-dMRI S0 was reconstructed both using LLS and MLE with known T2*. The ME-FLASH T2* map was included as a ground truth estimation for the MLE reconstruction. The noise distribution was estimated from the acquired noise map, using a recently published method to describe multi-coil data7. The voxel-wise SNR gain factor of reconstructing multiple echoes was calculated using Eq1.
Results
Simulations: In contrast to LLS, MLE shows an unbiased reconstruction of S0. LLS induces signal biases, especially at low SNR values, due to its assumption of Gaussian noise (Fig2). When including known T2*, MLE reconstructions appear sharply centered around the ground-truth S0. In contrast, LLS reconstructions become more biased when T2* knowledge is incorporated into the model fit. Simulations suggest that employing MLE (with known T2*) in ME-dMRI reconstructions is favorable due to its ability to deal with low SNR values of S0.MR-Reconstructions: The LLS and MLE reconstructions support the simulation results, showing that MLE is able to prevent signal bias in reconstructions of small S0 values. The reconstruction histograms show similar results for LLS and MLE in high-signal regions, but severe differences for low signals (Fig4). The mode SNR gains for optimally combined ME-dMRI reconstructions compared to single-echo acquisitions were ~1.4 for white matter (WM) and ~1.8 for grey matter (GM), respectively (Fig4).
Discussion
In dMRI, accurate and unbiased measurements of signal attenuation are key to reconstruct fiber orientations of diffusion models with high accuracy8. Computing an unbiased signal from multiple measurements (e.g., averages, directions, echoes) becomes increasingly challenging due to the emerging noise-floor of low SNR data. We show that noise-informed statistical data modeling can achieve ME-dMRI reconstructions with increased SNR and reduced signal bias. These benefits came at a small price of only a ~30% increase in scan time compared to single-echo acquisitions. This approach is not only applicable in the context of dMRI sEPI acquisitions. We expect MLE reconstructions of ME data to yield similar benefits in other imaging modalities such as steady-state-dMRI1, multi-dimensional diffusion-weighted-relaxometry9,10 or ME reconstructions for multi-parametric-mapping11.Acknowledgements
CE is supported by the SPP2041 program "Computational Connectomics" of the German Research Foundation (DFG) and the Max Planck Society.
MP is supported by a scholarship (PDF-502732-2017) from the Natural Sciences and Engineering Research Council of Canada (NSERC).
References
1. Miller KL et al. Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage. 2011.
2. Dyrby TB et al. An ex vivo imaging pipeline for producing high‐quality and high‐resolution diffusion‐weighted imaging datasets. Hum Brain Mapp. 2011.
3. Eichner C et al. Multi-echo segmented diffusion-weighted MRI for ex-vivo whole-brain measurements with 300mT/m gradients. ISMRM 2019.
4. Kundu P et. al. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage. 2012.
5. Sijbers J et al. Maximum-likelihood estimation of Rician distribution parameters. IEEE Trans Med Imag. 1998.
6. Feinberg DA, Oshio K. Phase errors in multi-shot echo planar imaging. Magn Reson Med. 1994.
7. St-Jean S et al. Automated characterization of noise distributions in diffusion MRI data. bioRxiv 2019.
8. Eichner C et al. Real diffusion-weighted MRI enabling true signal averaging and increased diffusion contrast. Neuroimage. 2015.
9. Hutter J et al. Integrated and efficient diffusion-relaxometry using ZEBRA. Sci Rep. 2018.
10. Tax CMW et al. Characterizing diffusion of myelin water in the living human brain using ultra-strong gradients and spiral readout. ISMRM 2019.
11. Weiskopf N. et al. Estimating the apparent transverse relaxation time (R2*) from images with different contrasts (ESTATICS) reduces motion artifacts. Front Neurosci. 2014.
Figures