4489
SlicerDMRI: a suite of clinician-accessible tools for neurosurgical planning research using diffusion MRI and tractography
Fan Zhang1, Thomas Noh1, Parikshit Juvekar1, Sarah F Frisken1, Laura Rigolo1, Isaiah Norton1, Tina Kapur1, Sonia Pujol1, William Wells III1,2, Alex Yarmarkovich3, Gordon Kindlmann4, Demian Wassermann5, Raul San Jose Estepar1, Yogesh Rathi1, Ron Kikinis1,6, Hans J Johnson7, Carl-Fredrik Westin1, Steve Pieper3, Alexandra J Golby1, and Lauren J O'Donnell1
1Harvard Medical School, Boston, MA, United States, 2Massachusetts Institute of Technology, Boston, MA, United States, 3Isomics, Inc., Cambridge, MA, United States, 4University of Chicago, Chicago, IL, United States, 5Université Paris-Saclay, Palaiseau, France, 6University of Bremen and Fraunhofer MEVIS, Bremen, Germany, 7University of Iowa, Iowa City, IA, United States
1Harvard Medical School, Boston, MA, United States, 2Massachusetts Institute of Technology, Boston, MA, United States, 3Isomics, Inc., Cambridge, MA, United States, 4University of Chicago, Chicago, IL, United States, 5Université Paris-Saclay, Palaiseau, France, 6University of Bremen and Fraunhofer MEVIS, Bremen, Germany, 7University of Iowa, Iowa City, IA, United States
Synopsis
We present an open-source software suite, SlicerDMRI (dmri.slicer.org), that enables neurosurgical planning research using diffusion magnetic resonance imaging (dMRI). SlicerDMRI is built upon and deeply integrated with 3D Slicer, an NIH-supported open-source platform for medical image informatics, image processing, and three-dimensional visualization. In this work, we give a demonstration of SlicerDMRI to enable end-to-end dMRI analyses in two retrospective imaging datasets from patients with high-grade glioma. Analyses demonstrated here include conventional diffusion tensor imaging (DTI) analysis, advanced multi-fiber tractography, automated identification of critical fiber tracts, and integration of multimodal imagery with dMRI.
Introduction
We present an open-source software suite, SlicerDMRI (dmri.slicer.org), that enables neurosurgical planning research using diffusion magnetic resonance imaging (dMRI). dMRI measures the random molecular diffusion of water molecules and provides the only non-invasive, in-vivo technique to investigate the organization of the brain white matter (WM)1. In neurosurgical planning research, brain mapping using dMRI can provide critical preoperative information2,3, including preoperative maps of brain WM connections and insight into the tissue microstructure surrounding tumors. Briefly speaking, dMRI enables a computational process, called tractography4 to trace WM connections such as critical motor, language, visual, and somatosensory tracts. Visualization of the relationship between the lesion and these tracts can be used for preoperative planning and intraoperative neuronavigation2,5–7. dMRI also provides quantitative microstructure measures, such as fractional anisotropy (FA) and mean diffusivity (MD), that allow assessment of changes of tissue microstructure caused by tumors and the effects of treatment8,9.SlicerDMRI enables the analysis and visualization of dMRI data and is aimed at the needs of clinical research users. SlicerDMRI is built upon and deeply integrated with 3D Slicer, an NIH-supported open-source platform for medical image informatics, image processing, and three-dimensional visualization10. Integration with 3D Slicer provides many features of interest to cancer researchers, such as real-time integration with neuronavigation equipment, intraoperative imaging modalities, and multimodal data fusion. SlicerDMRI has an average of over 200 downloads per month. The main functionality of SlicerDMRI includes dMRI visualization, interactive and automated tractography, tissue microstructure modeling, and quantitative analyses (see our previous paper11 for a technical overview). One key application of SlicerDMRI is in neurosurgery research, where brain mapping using dMRI can provide patient-specific maps of critical brain connections, as well as insight into the tissue microstructure surrounding brain tumors.
In this work, we focus on a demonstration of SlicerDMRI to enable end-to-end dMRI analyses in two retrospective imaging datasets from patients with high-grade glioma. Analyses demonstrated here include conventional diffusion tensor imaging (DTI)12 analysis, advanced multi-fiber tractography, automated identification of critical fiber tracts, and integration of multimodal imagery with dMRI.
Methods
Retrospective imaging datasets from two neurosurgical patients were selected to illustrate two different clinical scenarios: Patient-1 (69y, male) with a recurrent left frontoparietal high-grade glioma and significant surrounding edema displacing the corticospinal tracts (CST), and Patient-2 (49y, male) with a left temporoparietal high-grade glioma abutting the arcuate fasciculus (AF). Preservation of these tracts is critical for voluntary motor movements (CST)13, and language function (AF)14. All imaging data, including dMRI, anatomical T2w, gadolinium-enhanced T1w, ultrasound and functional MRI (fMRI) were acquired at Brigham and Women's Hospital, Boston, USA. The usage of the data was approved by the Partners Healthcare Institutional Review Board, and informed consent was obtained from the patients prior to scanning. All data analyses in the present work were performed retrospectively for research purposes only.We first give two examples of clinical applications using conventional DTI analysis in SlicerDMRI for neurosurgical planning research. We then use the same cases to highlight advanced dMRI analyses as well as its incorporation into multi-modal analysis combined with intraoperative ultrasound.
Results and discussion
Figure-1 demonstrates the first DTI-based application for visualization and quantification of tissue microstructure in Patient-1 to give insight into tissue microstructure differences between the tumor and surrounding tissues. Tumor-related edema and mass effect have distorted the local anatomy including the FA map.Figure-2 demonstrates the second DTI-based application for the identification of patient-specific fiber tracts in Patient-1 to inform surgical planning in order to minimize morbidity associated with disruption of critical white matter connections. The mean FA of the identified CST in the tumor-bearing hemisphere is lower than that in the healthy hemisphere, likely related to the tumor-related edema and/or infiltration.
Figure-3 demonstrates the first advanced dMRI application is based on multi-fiber Unscented Kalman Filter (UKF) tractography15 that provides an expanded range of multi-fiber models (including multi-tensor and multi-compartment models16,17) and enables more sophisticated mathematical modeling of the white matter tissue than single-tensor DTI tractography18–21. The UKF method with the free water model traces an apparently larger volume of fibers within the edema surrounding the tumor.
Figure-4 demonstrates the second advanced dMRI application is based on an anatomically curated white matter atlas22 and a data-driven fiber clustering pipeline22–24 to enable automated identification of critical fiber tracts in Patient-1. Six major language white matter connections are identified from the whole brain tractography computed using the 2-tensor UKF method with the free water model.
Figure-5 demonstrates a demonstration of how SlicerDMRI enables a multi-modal analysis of dMRI combined with intraoperative ultrasound data in Patient-2. The ultrasound information demonstrates brain shift that occurs after dural opening. By integrating this brain shift information with tractography, the surgeon can make an updated estimate of how far the tracts might be25,26. The intraoperative ultrasound image is used retrospectively here for a demonstration of software functionality.
Conclusion
SlicerDMRI provides open-source and clinician-accessible software tools for dMRI research. In this work, we demonstrate SlicerDMRI functionality as an informatics tool to enable end-to-end diffusion MRI analysis. We provide examples that demonstrate the usage of SlicerDMRI to retrospectively analyze data from brain tumor resection.Acknowledgements
The authors gratefully acknowledge the support of NIH NCI ITCR grant U01CA199459 (Open Source Diffusion MRI Technology For Brain Cancer Research), P41EB015898 (National Center for Image Guided Therapy, NCIGT), P41EB015902 (Neuroimaging Analysis Center, NAC), R01MH119222, R01CA235589, R01EB027134, and HHSN261200800001E. We are also thankful for other grant support over the lifetime of 3D Slicer, including NIH R01MH074794, R01MH097979, and U54EB005149 (National Alliance for Medical Image Computing, NA-MIC).References
1. Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy, and imaging. Biophys. J. 66, 259–267 (1994).2. Essayed, W. I. et al. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. Neuroimage Clin 15, 659–672 (2017).
3. Wu, J.-S. et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 61, 935–48; discussion 948–9 (2007).
4. Basser, P. J., Pajevic, S., Pierpaoli, C., Duda, J. & Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 44, 625–632 (2000).
5. Panesar, S. S. et al. Tractography for Surgical Neuro-Oncology Planning: Towards a Gold Standard. Neurotherapeutics 16, 36–51 (2019).
6. Nimsky, C., Bauer, M. & Carl, B. Merits and Limits of Tractography Techniques for the Uninitiated. Adv. Tech. Stand. Neurosurg. 37–60 (2016).
7. O’Donnell, L. J. et al. Automated white matter fiber tract identification in patients with brain tumors. Neuroimage Clin 13, 138–153 (2017).
8. Beppu, T. et al. Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg. Neurol. 63, 56–61; discussion 61 (2005).
9. Mori, S. et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann. Neurol. 51, 377–380 (2002).
10. Fedorov, A. et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 30, 1323–1341 (2012).
11. Norton, I. et al. SlicerDMRI: Open Source Diffusion MRI Software for Brain Cancer Research. Cancer Res. 77, e101–e103 (2017).
12. Pierpaoli, C., Jezzard, P., Basser, P. J., Barnett, A. & Di Chiro, G. Diffusion tensor MR imaging of the human brain. Radiology 201, 637–648 (1996).
13. Al Masri, O. An essay on the human corticospinal tract: history, development, anatomy, and connections. Neuroanatomy 10, 1–4 (2011).
14. Dick, A. S. & Tremblay, P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain 135, 3529–3550 (2012).
15. Malcolm, J. G., Shenton, M. E. & Rathi, Y. Filtered multitensor tractography. IEEE Trans. Med. Imaging 29, 1664–1675 (2010).
16. Pasternak, O., Sochen, N., Gur, Y., Intrator, N. & Assaf, Y. Free water elimination and mapping from diffusion MRI. Magn. Reson. Med. 62, 717–730 (2009).
17. Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61, 1000–1016 (2012).
18. Baumgartner, C. et al. A unified tractography framework for comparing diffusion models on clinical scans. in Computational Diffusion MRI Workshop of MICCAI, Nice 27–32 (2012).
19. Chen, Z. et al. Reconstruction of the arcuate fasciculus for surgical planning in the setting of peritumoral edema using two-tensor unscented Kalman filter tractography. Neuroimage Clin 7, 815–822 (2015).
20. Gong, S. et al. Free water modeling of peritumoral edema using multi-fiber tractography: Application to tracking the arcuate fasciculus for neurosurgical planning. PLoS One 13, e0197056 (2018).
21. Chen, Z. et al. Corticospinal tract modeling for neurosurgical planning by tracking through regions of peritumoral edema and crossing fibers using two-tensor unscented Kalman filter tractography. Int. J. Comput. Assist. Radiol. Surg. 11, 1475–1486 (2016).
22. Zhang, F. et al. An anatomically curated fiber clustering white matter atlas for consistent white matter tract parcellation across the lifespan. Neuroimage 179, 429–447 (2018).
23. O’Donnell, L. J., Wells, W. M., 3rd, Golby, A. J. & Westin, C.-F. Unbiased groupwise registration of white matter tractography. Med. Image Comput. Comput. Assist. Interv. 15, 123–130 (2012).
24. O’Donnell, L. J. & Westin, C.-F. Automatic tractography segmentation using a high-dimensional white matter atlas. IEEE Trans. Med. Imaging 26, 1562–1575 (2007).
25. Machado, I. et al. Non-rigid registration of 3D ultrasound for neurosurgery using automatic feature detection and matching. Int. J. Comput. Assist. Radiol. Surg. 13, 1525–1538 (2018).
26. Machado, I. et al. Deformable MRI-Ultrasound registration using correlation-based attribute matching for brain shift correction: Accuracy and generality in multi-site data. Neuroimage 202, 116094 (2019).
Figures
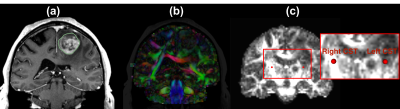
Figure-1: DTI visualization and quantification in Patient-1. The tumor-related edema and mass effect have distorted the local anatomy including the FA map. (a) T1w with a contrast-enhancing lesion and tumor region (in green outline) in the left frontal gyrus; (b) Visualization of DTI image using a directionally-encoded color map; (c) FA map with two fiducials in the CST (see the zoomed-in region), where the FA value from the tumor-bearing hemisphere is 0.34 and that from the healthy hemisphere is 0.84.
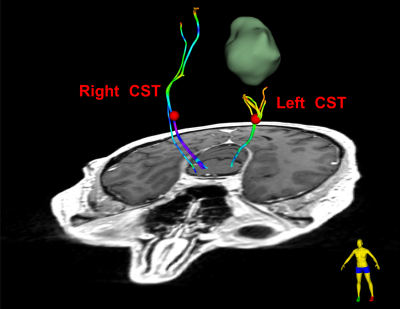
Figure-2: DTI tractography analysis for mapping white matter connections in Patient-1. DTI fiber tracking is seeded from the 3D fiducials that are placed in the CST bundles (specifically in the posterior limb of the internal capsule in each hemisphere, as indicated using the red sphere). The fiber tracts are colored by the FA values of the fiber points along the fiber tract. The mean FA of the fiber tract is 0.48 in the healthy hemisphere and 0.39 in the tumor-bearing hemisphere. The decreased FA is likely related to the tumor-related edema and/or infiltration.
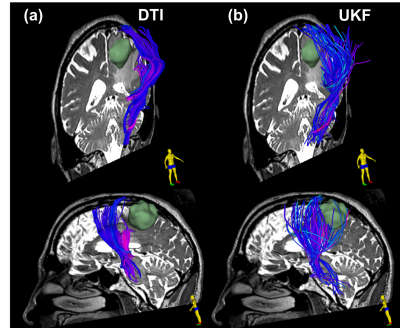
Figure-3: Single-tensor DTI vs two-tensor UKF tractography with a free water model of edema in Patient-1. The tracts are colored by the mean fiber orientation. The lateral projections of the CST that cross through edema and intersect with long anterior-posterior fibers are difficult to trace with single-tensor DTI (a) but can be traced using two-tensor UKF (b). The UKF method with the free water model traces an apparently larger volume of fibers within the edema surrounding the tumor.
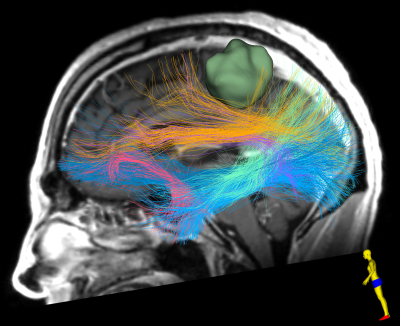
Figure-4: Automated identification of critical fiber tracts including major language WM connections in Patient-1. 2-tensor UKF method with the free water model is used for computing the whole brain tractography data. 6 language fiber tracts are identified and displayed, including the arcuate (purple), the inferior occipito-frontal (dark blue), the middle longitudinal (light green), the superior longitudinal (yellow), the uncinate (pink), and the inferior longitudinal (light blue) fasciculus.
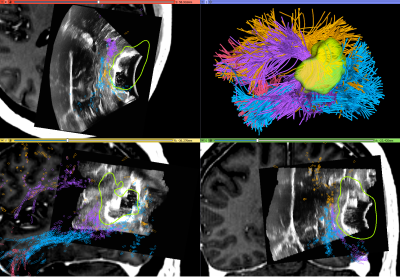
Figure-5: Visualization of multi-modal dMRI and ultrasound data in Patient-2. Navigated intraoperative ultrasound data is overlaid on pre-operative T1w MRI with contrast, along with the automatically identified language fiber tracts (tracts and colors as in Figure-4). iUS information demonstrates brain shift that occurs after dural opening and likely displaces the arcuate fasciculus (purple) which can be seen at the edge of various parts of the resection cavity in each of the panels.