4486
Advantage of readout-segmented EPI in simultaneous multi-slice diffusion MRI measurements for identifying uncinate fasciculus1Center for Information and Neural Networks, National Institute of Information and Communications Technology, Suita, Japan, 2Graduate School of Frontier Biosciences, Osaka University, Suita, Japan, 3Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, 4Siemens Healthcare K.K., Tokyo, Japan
Synopsis
We evaluated the performance of diffusion MRI (dMRI) acquisition with simultaneous multi-slice (SMS) readout-segmented echo-planar imaging (rs-EPI) in measuring the uncinate fasciculus, which is typically affected by susceptibility-induced distortion or signal dropout. We found that SMS rs-EPI data provides a larger number of streamlines supported by dMRI data and larger fractional anisotropy in uncinate, as compared to conventional SMS single-shot EPI data. However, such an advantage was not consistent across subjects in the forceps major. Taken together, our results demonstrate that dMRI acquisition with SMS rs-EPI has advantages in measuring specific white matter tracts affected by susceptibility-induced artifacts.
Introduction
Diffusion-weighted magnetic resonance imaging (dMRI) and tractography are essential methods to evaluate properties of white matter tracts in living humans (Assaf et al., 2019). However, conventional single-shot echo-planar imaging (ss-EPI) used for dMRI has limitations derived from susceptibility-induced image distortions and signal dropout. This limitation poses challenges for identifying the full extent of the uncinate fasciculus (Von Der Heide et al., 2013), which is located in areas affected by susceptibility-induced artifacts. Readout-segmented EPI (rs-EPI; Porter & Heidemann, 2009) acquisitions could overcome the limitations but the disadvantage is a prolonged scan time (Kida et al., 2016). Recently, the rs-EPI has incorporated a simultaneous multi-slice (SMS) excitation to substantially reduce the scan time (Frost et al., 2015). However, it is not yet understood in which anatomical structure dMRI measurement using SMS rs-EPI has an advantage compared to when using SMS ss-EPI. In this study, we evaluated the advantage of SMS rs-EPI as compared with SMS ss-EPI for measuring the human uncinate fasciculus.Methods
Data acquisition: Five healthy subjects (mean age: 23.8, all males) underwent MRI sessions with written informed consent. Data were acquired using a 3T SIEMENS Prisma scanner with a 32-channel head coil. We collected dMRI data using rs-EPI (prototype sequence, TE: 53 ms, TR: 4390 ms; number of readout segments: 5; echo spacing: 0.34 ms) and ss-EPI (TE: 63 ms, TR: 4000 ms; echo spacing: 0.65 ms) with SMS excitation (SMS factor: 2) and in-plane acceleration (iPAT: 2). dMRI data were sampled in 30 directions at a spatial resolution of 2 mm isotropic (number of slices: 72). The b-value was set to 1000 s/mm2. For both SMS rs-EPI and SMS ss-EPI, four non-diffusion weighted images (b=0) were obtained per each scanning session. In addition, two b=0 images with reversed phase encoding direction was obtained for correcting susceptibility-induced distortions (Andersson et al., 2003). The data acquisition using SMS ss-EPI was repeated five times in order to match the scan duration with SMS rs-EPI (acquisition time for SMS rs-EPI: 13:11 min, five repetitions of SMS ss-EPI: 12:50 min). All subjects participated in MRI sessions over two different days, in order to evaluate reproducibility of the measurements.Data analysis: dMRI images were corrected for susceptibility-induced distortions and eddy current distortions using FSL (Andersson and Sotiropoulos, 2016). ss-EPI data were averaged across five acquisition sessions. We fit diffusion tensor model to dMRI data using RESTORE (Chang et al., 2005) to estimate fractional anisotropy (FA) in each voxel. We performed tractography by using constrained spherical deconvolution-based probabilistic tractography (MRTrix; Tournier et al., 2012). Streamline generation was performed at five different angle thresholds (Takemura et al., 2016). We discarded streamlines that did not contribute to diffusion signal prediction using Linear Fascicle Evaluation (LiFE; Pestilli et al., 2014). Finally, we identified the uncinate fasciculus using Automated Fiber Quantification (Yeatman et al., 2012).
Results
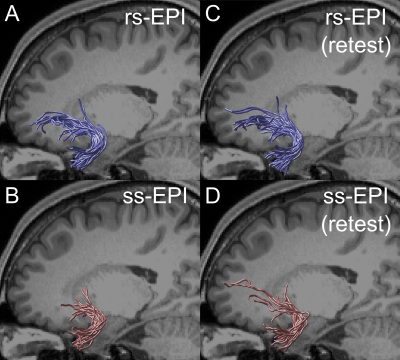
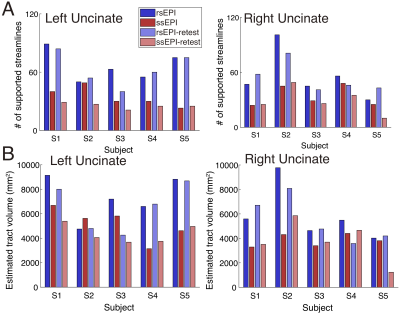
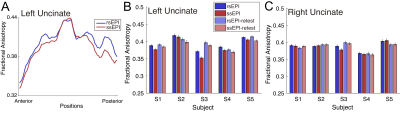
Figure 1A depicts uncinate streamlines identified from the SMS rs-EPI (upper panel) and SMS ss-EPI dataset (lower panel). Uncinate streamlines identified from SMS rs-EPI reached the anterior part of the orbitofrontal cortex (OFC), while streamlines identified from SMS ss-EPI did not. This difference was qualitatively replicated in the retest dataset acquired on a different day (Figure 1C-D). In all hemispheres, a number of uncinate streamlines supported by LiFE were consistently larger in SMS rs-EPI, as compared with SMS ss-EPI in both test and retest datasets (Figure 2A). The estimated tract volume of the uncinate was larger in SMS rs-EPI in a majority of hemispheres (9 of 10 in both test and retest dataset; Figure 2B).We also evaluated diffusivity measurement (FA) along the uncinate between those sequences. We found that FA along the left uncinate was higher in SMS rs-EPI compared to SMS ss-EPI in all subjects (Figure 3A-B; mean FA ± 1 s.d. across subjects: 0.394 ± 0.020, 0.384 ± 0.025 for SMS rs-EPI, SMS ss-EPI, respectively). We did not find evidence of FA difference in the right uncinate (Figure 3C; mean FA ± 1 s.d. across subjects: 0.387 ± 0.013, 0.385 ± 0.015 for SMS rs-EPI, SMS ss-EPI, respectively).
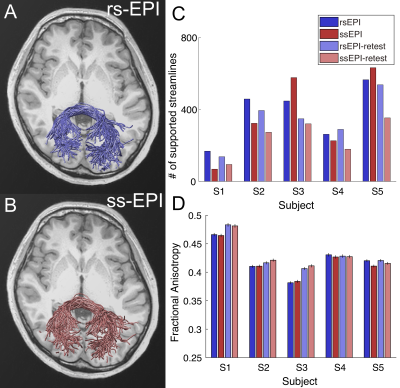
Finally, we analyzed the forceps major, which is a callosal pathway in the occipital lobe and likely to be less affected by susceptibility artifacts. Unlike the uncinate, differences in a number of supported streamlines and FA in the forceps major between those sequences were not consistent across subjects or test-retest datasets (Figure 4).
Discussion
The results of SMS rs-EPI indicate the projections of the uncinate fasciculus to the anterior OFC, which are known to exist in the macaque brain by an invasive tracer study (Schmahmann & Pandya, 2006). Measuring the anterior OFC projections of the uncinate fasciculus by using in vivo dMRI will provide an avenue to evaluate the properties and dysfunctions of white matter pathways mediating information transmission between the human OFC and limbic system. Our results demonstrated that SMS rs-EPI has an advantage in measuring such white matter connections, which is sensitive to susceptibility artifacts.Conclusion
This study demonstrates that dMRI acquisition with SMS rs-EPI has advantages in measuring specific white matter tracts, such as the uncinate fasciculus, in which dMRI data acquisitions are affected by susceptibility-induced image distortion and signal dropout in conventional SMS ss-EPI acquisitions.Acknowledgements
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (17H04684, H.T). We thank Yusuke Sakai in support for data acquisition and analysis.References
Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 2003; 20(2):870-888.
Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 2016; 125:1063-1078.
Assaf Y, Johansen‐Berg H, Thiebaut de Schotten M. The role of diffusion MRI in neuroscience. NMR Biomed. 2019; 32(4):e3762.
Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med.2005; 53(5):1088-1095.
Frost R, Jezzard P, Douaud G et al. Scan time reduction for readout-segmented EPI using simultaneous multislice acceleration: Diffusion-weighted imaging at 3 and 7 Tesla. Magn Reson Med.2015; 74(1):136-149.
Kida I, Ueguchi T, Matsuoka Y, et al. Comparison of diffusion-weighted imaging in the human brain using readout-segmented EPI and PROPELLER turbo spin echo with single-shot EPI at 7 T MRI. Invest Radiol. 2016; 51(7):435-439.
Pestilli F, Yeatman JD, Rokem A, et al. Evaluation and statistical inference for human connectomes. Nat Methods 2014; 11(10):1058.
Porter DA, & Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med.2009; 62(2):468-475.
Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford University Press.
Takemura H, Caiafa CF, Wandell BA, et al. Ensemble tractography. PLoS Comput Biol., 2016; 12(2):e1004692.
Tournier JD, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol., 2012; 22(1):53-66.
Von Der Heide RJ, Skipper LM, Klobusicky E, et al. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain, 2013; 136(6):1692-1707.
Yeatman JD, Dougherty RF, Myall NJ, et al. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS ONE, 2012; 7(11):e49790.
Figures