4480
Investigating age-related differences in fractional anisotropy in relation to complex fibre architecture1Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Departments of Psychiatry and Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
It is well established that white matter diffusion fractional anisotropy (FA) is sensitive to age, but interpreting this effect in terms of cellular microstructure has posed a challenge. Here we investigate how age-related differences in FA relate to measures of crossing and dispersing fibres derived from advanced diffusion MRI models. We find that, in aging, decreased FA is associated with increased fibre dispersion, and increased FA is associated with selective degeneration of crossing fibres. Importantly, we show that age-related differences in FA more closely reflect age-related differences in fibre architecture when applying free water elimination.
Introduction
It is well established that white matter diffusion fractional anisotropy (FA) is sensitive to age, but the non-specific nature of FA poses a challenge in interpreting this effect in terms of cellular microstructure, especially in the presence of complex fibre architecture.1 NODDI studies, using multi-shell data, suggest that decreased FA with age is due in part to increased fibre dispersion with age.2-3 Also, in studies of high statistical power, increased FA with age has been found in regions of crossing fibres, interpreted as selective degeneration of a secondary tract while the primary tract remains intact.3-4 It has been suggested that this ‘increased FA with age’ effect can be recovered in studies of more moderate sample sizes by separating the effect of cellular diffusion from the effect of extracellular isotropic water movement by modeling the latter as free water (FW).5The purpose of the current study is to investigate how closely FA reflects multi-shell measures of fibre dispersion and crossings in aging, and to test the hypothesis that free-water eliminated (cellular) FA (FAc) is more reflective of fibre architecture than conventional FA.
Methods
Study ParticipantsAll subjects were participants of the UK Biobank. Subjects were excluded from our study if they reported a neurological, psychological or psychiatric disorder, neurological injury, or history of stroke. Subjects were divided into seven age categories [46-50, 51-55, 56-60, 61-65, 66-70, 71-75, 76-80] years and 100 subjects were randomly selected from each of the seven age categories. Data from 52 of the subjects were deemed unusable by the UK Biobank, and 4 additional data were deemed unusable by our own quality control. The final sample thus consisted of 644 subjects aged 46-80 (54% male, 46% female).
Diffusion MRI Data
Information about dMRI data and processing can be found at biobank.ctsu.ox.ac.uk/crystal/crystal/docs/brain_mri.pdf. In brief, dMRI data were acquired on a Siemens Skyra 3T with 5x b=0, 50x b=1000, 50x b=2000 s/mm2 and corrected for distortions via TOPUP.6 The NODDI model was fit to all diffusion MRI data using the AMICO toolbox.7 The proportions of crossing fibre tracts were estimated as the posterior mean fractional voxel occupancy of each tract using bedpostX.8 DTI with and without free water elimination was fit to the b=0 and b=1000s/mm2 data using in-house MATLAB code.9
Data Analysis
FSL’s Tract-Based Spatial Statistics10 was used to obtain a WM skeleton. For voxelwise analysis, a relationship of metric with age defined by p<0.05 with correction for multiple comparisons (cluster-wise controlling family-wise error rate with 5000 permutations) and controlling for gender as per FSL’s randomise.11 Age-related difference of metric per year was calculated as the regression coefficient from a general linear model as implemented in FreeSurfer.
Results
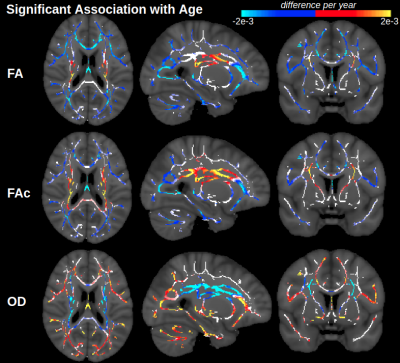
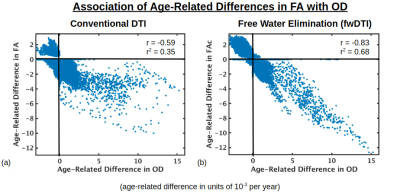
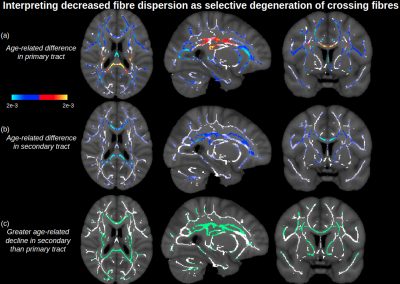
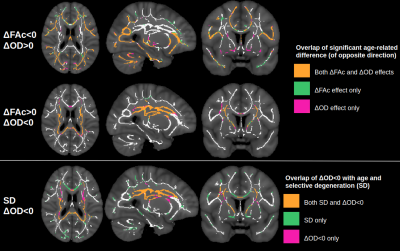
Figure 1 displays voxels with significant associations between FA and age, which, after FW elimination, correspond more closely with significant associations of NODDI-derived orientation dispersion (OD) and age. Specifically, across all voxels of the white matter skeleton, age-related difference per year in conventional FA (ΔFA) is correlated with both age-related difference in OD (ΔOD, r=-0.59) and age-related difference in FW (ΔFW, r=-0.43), but with FW eliminated, the correlation of ΔOD and ΔFAc strengthens (r=-0.83) (Figure 2).To help understand why there are some regions displaying negative associations of OD with age (and correspondingly positive associations of FAc with age), Figure 3 displays voxels with significant associations between age and the fractional voxel occupancy of primary (Figure 3a) and secondary (Figure 3b) tracts, modeled via bedpostX. Voxels showing greater age-related decline in the secondary tract than primary tract are highlighted in green (Figure 3c). Of the voxels in the WM skeleton displaying negative associations of OD with age, 79% show greater age-related decline in secondary tract than primary tract (Figure 4).
Discussion
This study suggests that age-related differences in FA reflect both differences in cellular OD and extracellular FW, and eliminating the effect of FW strengthens the relationship between cellular FA (FAc) and OD. While OD is positively associated with age in single-fibre populations, regions with negative associations of OD (or positive association of FAc) with age is likely due to selective degeneration of secondary crossing fibres. Note that these crossing fibre results, derived from a multi-shell implementation of bedpostX, were similar to a previous smaller study that only used a single shell of b=700.5Conclusion
This work emphasizes the importance of interpreting age-related differences in FA in relation to the underlying complex fibre architecture, suggesting that age-effects on FA reflect a combination of cellular fibre disorganization and elevated extracellular FW with age, and that insight into fibre disorganization can be disentangled by eliminating FW.Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 40922. We thank CIHR, NSERC and NIH for financial support.References
1. Madden DJ et al (2012) Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta 1822(3):386-400.
2. Billiet T et al (2015) Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging 36(6):2107-2121.
3. Miller et al (2016) Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19(11):1523-1536.
4. de Groot M et al (2016). White Matter Degeneration with Aging: Longitudinal Diffusion MR Imaging Analysis. Radiology 279(2):532–541.
5. Chad JA et al (2018) Re-examining age-related differences in white matter microstructure with free-water corrected diffusion tensor imaging. Neurobiol Aging 71:161-170.
6. Andersson JL et al (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20(2):870-888.
7. Daducci A et al (2015) Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage 105:32-44.
8. Jbabdi S et al (2012) Model-based analysis of multi-shell diffusion MR data for tractography: How to get over fitting problems. Magn Reson Med 68(6):1846-1855.
9. Pasternak O et al (2009) Free water elimination and mapping from diffusion MRI. Magn Reson Med 62(3):717-730.
10. Smith SM et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487-1505.
11. Winkler AM et al (2014) Permutation inference for the general linear model. Neuroimage 92:381-397.
Figures