4477
Gender differences in brain white matter discovered by whole brain suprathreshold fiber cluster statistics
Fan Zhang1, Suheyla Cetin-Karayumak1, Yang Song2, Weidong Cai3, James J Levitt1, Nikos Makris1, Carl-Fredrik Westin1, and Lauren J O'Donnell1
1Harvard Medical School, Boston, MA, United States, 2The University of New South Wales, Sydney, Australia, 3The University of Sydney, Sydney, Australia
1Harvard Medical School, Boston, MA, United States, 2The University of New South Wales, Sydney, Australia, 3The University of Sydney, Sydney, Australia
Synopsis
We propose to study whole-brain white matter connectivity differences between females and males using diffusion MRI (dMRI) tractography. We leverage a well-established data-driven fiber clustering pipeline and a novel suprathreshold fiber cluster statistical method. We study a large cohort of 707 healthy adult subjects from the Human Connectome Project. We find multiple fiber tracts that are significantly different between the female and male groups in terms of the fractional anisotropy and/or the trace measures of the fiber tracts. These tracts include the corticospinal tract, the arcuate fasciculus, and the corpus callosum tract.
Introduction
Gender differences have been of enduring scientific and societal interest. There is an evident prevalence of gender-biased conditions that are associated with the sexual dimorphism of the brain’s structural connections1. With the advent of diffusion MRI (dMRI), many studies have explored white matter (WM) structural differences between genders, in particular using the tractography technique2–8. While most tractography-based gender studies are hypothesis-driven (i.e. tract-of-interest-based), there are relatively few studies on whole-brain WM connectivity6–8. Studying the whole-brain connectivity is important to provide fundamental insights into the organization and integration of brain networks8. While the connectome-based method (i.e. subdividing the entire WM based on a cortical parcellation) is widely used to perform whole-brain connectivity analysis, many other studies have applied fiber clustering (i.e. grouping the WM fibers according to fiber geometric trajectories), which has been shown to be highly consistent and reliable for WM parcellation9–14.In this work, we propose to study whole-brain WM connectivity differences between females and males using dMRI tractography. We leveraged a data-driven fiber clustering pipeline15,16 and a whole-brain WM atlas10 that together enable whole-brain tractography parcellation into multiple fiber parcels, where fibers within each parcel have similar WM anatomy (i.e. similar fiber trajectory). We applied a recently proposed suprathreshold fiber cluster (STFC) method17 to test for statistical gender differences in the whole-brain WM. The STFC method that leverages the whole-brain fiber geometry to enhance statistical analyses of tractography while correcting for multiple comparisons to allow simultaneous analysis across the entire white matter17. We studied a large cohort of 707 healthy adult subjects from the Human Connectome Project (HCP)18. We found multiple white matter fiber tracts that show differences between females and males using the dMRI quantitative measures of fractional anisotropy (FA) and trace.
Methods
Study population: We used dMRI data (b=0/3000s/mm2, 90 directions, 18 b=0, TE/TR=89.5/5520ms, and 1.25x1.25x1.25mm3) from the healthy adult cohort in the Human Connectome Project (HCP)18. We performed the following subject selection procedures: 1) excluding one of a twin pair if both twins participated in the HCP study, 2) selecting the maximal subset of subjects that have matched ages between the male and female groups. In total, we used 707 subjects in our study, including 340 females and 367 males (mean age: 28.2±3.1y versus 28.0±3.6y, p=0.43).Tractography parcellation: Whole-brain tractography was computed for each subject using a multi-fiber Unscented Kalman Filter (UKF) method19 implemented in SlicerDMRI20. Fiber clustering was performed to parcellate the tractography data into 800 fiber parcels10,15,16. For each cluster, we extracted the mean FA and the mean trace measures along the fiber tracts. We chose FA and trace because they have been widely studied in previous work on gender differences2,21.
STFC statistical comparison: For each dMRI feature, the STFC method17 first identified suprathreshold parcels that passed an uncorrected, parcel-level two-tailed Student's t-test (p<0.05). Then the suprathreshold parcels were formed as STFCs, within which multiple suprathreshold parcels were neighboring to each other based on the fiber geometric similarity. A nonparametric permutation test was used to determine a corrected p value for each STFC based on its STFC size, i.e. the number of WM parcels, under the null hypothesis of no group difference in any parcel in this STFC. The STFCs with the corrected p<0.05 were considered to be significantly different between the two groups.
Results
Three STFCs were identified to be significantly different between female and male groups for the mean FA measure (Figure-1), including one STFC connecting the parietal lobe to the striatum in the left hemisphere, and bilateral STFCs connecting the corona radiata through the brain stem. For each identified STFC, the female group had a higher mean FA compared to the male group.Six STFCs were identified to be significantly different between the female and male groups for the mean trace measure (Figure-2), including two STFCs from the commissural corpus callosum (CC) tracts, one STFC from the left arcuate fasciculus (AF), one STFC from the left inferior occipito-frontal (IoFF) fasciculus, and bilateral STFCs connecting the corona radiata to the brain stem. For each identified STFC, the female group had a higher mean trace than the male group.
Discussion
We found bilateral tracts from the corona radiata to the brain stem, including the CSTs, to be significantly different between genders in both FA and trace. Multiple studies have reported gender-specific differences in these WM regions22–24. We also identified one significantly different tract related to the parietal lobe that has been shown to have gender differences in terms of the proportion of GM/WM volumes25. These results suggest potential microstructural differences in the projection WM connections.Two commissural tracts were significantly different in the corpus callosum, a region that has been reported to have gender differences26,27. Two association tracts in the left hemisphere were identified, including the AF and IoFF tracts that are related to human language functions28,29.
Conclusion
We studied whole-brain WM gender differences using a data-driven fiber clustering pipeple, a WM parcellation atlas, and a novel STFC statistical analysis method. We compared FA and trace between the two groups in a large cohort of 707 subjects. We identified multiple fiber tracts that had significant differences, which provides evidence pointing to the gender-specific brain WM connectivity.Acknowledgements
The authors gratefully acknowledge the support of the following grants: NIH P41EB015898, R01MH108574, P41EB015902, R01MH119222, U01CA199459, and UNSW USA Networks of Excellence Grant 2019.References
1. de Vries, G. J. & Södersten, P. Sex differences in the brain: the relation between structure and function. Horm. Behav. 55, 589–596 (2009).2. Lebel, C., Caverhill-Godkewitsch, S. & Beaulieu, C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage 52, 20–31 (2010).
3. Oh, J. S. et al. Tractography-guided statistics (TGIS) in diffusion tensor imaging for the detection of gender difference of fiber integrity in the midsagittal and parasagittal corpora callosa. NeuroImage 36, 606–616 (2007).
4. Choi, C.-H. et al. Sex differences in the temporal lobe white matter and the corpus callosum: a diffusion tensor tractography study. Neuroreport 21, 73–77 (2010).
5. Thiebaut de Schotten, M. et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage 54, 49–59 (2011).
6. Jahanshad, N. et al. Sex differences in the human connectome: 4-Tesla high angular resolution diffusion imaging (HARDI) tractography in 234 young adult twins. in 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro 939–943 (2011).
7. Yan, C. et al. Sex- and Brain Size–Related Small-World Structural Cortical Networks in Young Adults: A DTI Tractography Study. Cereb. Cortex 21, 449–458 (2011).
8. Ingalhalikar, M. et al. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. U. S. A. 111, 823–828 (2014).
9. Ziyan, U., Sabuncu, M. R., Grimson, W. E. L. & Westin, C.-F. Consistency Clustering: A Robust Algorithm for Group-wise Registration, Segmentation and Automatic Atlas Construction in Diffusion MRI. Int. J. Comput. Vis. 85, 279–290 (2009).
10. Zhang, F. et al. An anatomically curated fiber clustering white matter atlas for consistent white matter tract parcellation across the lifespan. Neuroimage 179, 429–447 (2018).
11. Ge, B. et al. Group-wise consistent fiber clustering based on multimodal connectional and functional profiles. Med. Image Comput. Comput. Assist. Interv. 15, 485–492 (2012).
12. Sydnor, V. J. et al. A comparison of three fiber tract delineation methods and their impact on white matter analysis. Neuroimage 178, 318–331 (2018).
13. Zhang, F. et al. Comparison between two white matter segmentation strategies: An investigation into white matter segmentation consistency. in 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017) 796–799 (2017).
14. Zhang, F. et al. Test-retest reproducibility of white matter parcellation using diffusion MRI tractography fiber clustering. Hum. Brain Mapp. 40, 3041–3057 (2019).
15. O’Donnell, L. J. & Westin, C.-F. Automatic tractography segmentation using a high-dimensional white matter atlas. IEEE Trans. Med. Imaging 26, 1562–1575 (2007).
16. O’Donnell, L. J., Wells, W. M., III, Golby, A. J. & Westin, C.-F. Unbiased Groupwise Registration of White Matter Tractography. in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2012 (eds. Ayache, N., Delingette, H., Golland, P. & Mori, K.) 123–130 (Springer Berlin Heidelberg, 2012).
17. Zhang, F. et al. Suprathreshold fiber cluster statistics: Leveraging white matter geometry to enhance tractography statistical analysis. Neuroimage 171, 341–354 (2018).
18. Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013).
19. Malcolm, J. G., Shenton, M. E. & Rathi, Y. Filtered multitensor tractography. IEEE Trans. Med. Imaging 29, 1664–1675 (2010).
20. Norton, I. et al. SlicerDMRI: Open Source Diffusion MRI Software for Brain Cancer Research. Cancer Res. 77, e101–e103 (2017).
21. Szeszko, P. R. et al. Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport 14, 2469–2473 (2003).
22. Hervé, P.-Y. et al. Handedness, motor skills and maturation of the corticospinal tract in the adolescent brain. Hum. Brain Mapp. 30, 3151–3162 (2009).
23. Poletti, S., Melloni, E., Mazza, E., Vai, B. & Benedetti, F. Gender-specific differences in white matter microstructure in healthy adults exposed to mild stress. Stress 1–9 (2019).
24. Bava, S. et al. Sex differences in adolescent white matter architecture. Brain Res. 1375, 41–48 (2011).
25. Koscik, T., O’Leary, D., Moser, D. J., Andreasen, N. C. & Nopoulos, P. Sex differences in parietal lobe morphology: relationship to mental rotation performance. Brain Cogn. 69, 451–459 (2009).
26. Menzler, K. et al. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage 54, 2557–2562 (2011).
27. Luders, E. & Toga, A. W. Chapter 1 - Sex differences in brain anatomy. in Progress in Brain Research (ed. Savic, I.) 186, 2–12 (Elsevier, 2010).
28. Kang, X., Herron, T. J. & Woods, D. L. Regional variation, hemispheric asymmetries and gender differences in pericortical white matter. Neuroimage 56, 2011–2023 (2011).
29. Coates, J. Women, men and language: A sociolinguistic account of gender differences in language. (Routledge, 2015).
Figures
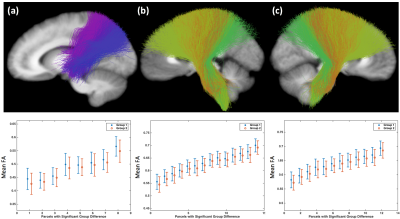
Figure-1: Three STFCs are identified to be significantly different between the female (Group 1) and male (Group 2) groups in terms of the mean FA measure, including (a) from the parietal lobe to the striatum in the left hemisphere, (b) from the left the corona radiata through the brain stem, and (c) from the right corona radiata through the brain stem. The bar plot shows the mean and std of the mean FA of each WM parcel within the STFCs across all subjects per group.
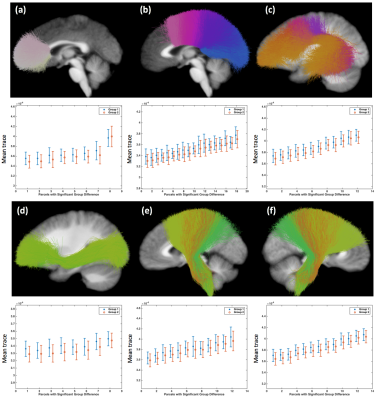
Figure-2: Six STFCs are identified to be significantly different between the female (Group 1) and male (Group 2) groups in terms of the mean trace measure, including (a) the anterior corpus callosum tract, (b) the middle and posterior corpus callosum tract, (c) the left arcuate fasciculus, (d) the left inferior occipito-frontal fasciculus, (e) from the left the corona radiata through the brain stem, and (f) from the right corona radiata through the brain stem. The bar plot shows the mean and std of the mean trace of each WM parcel within the STFCs across all subjects per group.