4466
Investigating SLFI anatomy using multi-resolution dMRI
Chiara Maffei1, Robert Jones1, Connor Johnson2, Hui Wang1, and Anastasia Yendiki1
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Brown University, Providence, RI, United States
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Brown University, Providence, RI, United States
Synopsis
The ability of tractography methods to discern between different fiber populations in the presence of challenging anatomical architectures remains limited, and it is hampered by the low spatial resolution achievable by diffusion MRI (dMRI). As a result, tractography often fails to reliably reconstruct some major white matter connections of the human brain. Here we incorporate high-resolution ex-vivo dMRI into the tractography process to understand whether this additional information can improve tractography results.
Introduction
The SLF is a major fronto-parietal cortico-cortical association pathway with three subcomponents (SLFI, SLFII, SLFIII) organized in a medio-to-lateral and dorso-to-ventral fashion1. The human morphology of the most dorsal component (SLFI) remains controversial, and its tractography-based reconstruction challenging. Particularly, while the literature agrees on its posterior terminations in the superior parietal lobe (SPL) and precuneus, it remains unclear whether the SLFI terminates in the rostral part of the primary motor cortex2, or it extends more anteriorly to connect regions in the superior frontal gyrus (SFG)3. As frontal pathways have been recently associated with cognitive control, a better understanding of the anatomy of the SLFI could help the study of this connection in psychopathological conditions such as attention deficit hyperactivity disorder, obsessive compulsive behaviour, and schizophrenia4. Here we investigate whether we can inform the anatomical definition of such more challenging bundles by integrating the information coming from other higher-resolution data.Methods
Ex-vivo dMRI: The samples used in this study were extracted from the left hemisphere of a human brain, which had been obtained from the Massachusetts General Hospital 160 Autopsy Suite and fixed in 10% formalin for at least two months. The fixed hemisphere had previously received a scan in a 3T Siemens Trio scanner, using a product 32-channel head coil and a 3D diffusion-weighted steady state free precession (DW-SSFP) sequence with TR=30.21 ms, TE=25.12 ms, 750 𝜇m isotropic resolution, 8 𝑏=0 volumes, and 60 DW volumes (𝑏=4,000 s/mm2, 𝛿=15 ms and Δ=19 ms). From this hemisphere we cut 3 small blocks (roughly 2x2x1cm) starting from the and running along the SFG (Figure 1). These were successively scanned in a small-bore 4.7T Bruker scanner using 3D EPI, (0.7x0.7x0.7mm, TR=750ms, TE=43ms, 𝛿=15ms, Δ=19ms, maximum b=40,000s/mm2), with 515 volumes corresponding to a Cartesian lattice in q-space. These data were resampled on q-space shells(s/mm2) of the HCP scheme, using a fast implementation of the non-uniform fast Fourier transform (NUFFT)5. Tractography: CSD as implemented in MRtrix3 was fit to both the low-res data and to the high-res resampled data. Fiber orientation functions (FOD) were obtained using i) an iterative response function estimation only on the highest shell6 and ii) tissue-specific manually estimated response functions on all the shells. In order to integrate the information from both the low- and high-res data, a MultiResolution tractography algorithm was implemented starting from the open-source probabilistic tractography code available in DiPy7.Results
Figure 2 shows the results of incorporating the high-resolution information in the tractography reconstruction of the SLFI, using MultiResolution tractography. Compared to the results obtained when using the same tractography algorithm, but without the high-resolution information, the SLFI reconstruction shows more streamlines connecting the posterior third of the SFG (block 3 in figure 1) and recovers streamlines connecting more frontal regions (block 2) that were not visible in the regular tractography reconstruction. This improvement in accuracy reconstruction seems to be consistent across different FODs reconstructions.Discussion and Conclusion
We have previously shown that, by using models of white-matter pathways labeled manually on high-quality in vivo dMRI as training data for global probabilistic tractography with anatomical priors, we can improve automated reconstruction of the same pathways in lower-quality in vivo dMRI8. However, even the highest quality in vivo dMRI is not sufficient to resolve some of the long-running controversies in connectional anatomy, such as the endings of the SLFI. Hence, we plan to use ground truth from optical imaging at microscopic resolutions, combined with post-mortem dMRI at mesoscopic resolutions, to build even more accurate models of white-matter pathways, which we will then use to train our automated reconstruction. The work presented here is a first step in the direction of combining data collected at multiple scales to advance knowledge of white-matter anatomy, which can in turn be used to improve automated tractography on routine-quality, in-vivo scans.Acknowledgements
Additional research support was provided by the National Institute of Biomedical Imaging and Bioengineering (R01-EB021265). Imaging was carried out at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41-EB015896, a P41 Biotechnology Resource Grant, and instrumentation supported by the NIH Shared Instrumentation Grant Program(S10RR025563, S10RR023401, S10RR019307, and S10RR023043)References
1..Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; 2006. 6. 2. Hecht EE, Gutman DA, Bradley BA, Preuss TM, Stout D. Virtual dissection and comparative connectivity of the superior longitudinal fasciculus in chimpanzees and humans. Neuroimage. 2014;108:124-37. 2.. Howells H, Thiebaut de Schotten M, Dell'Acqua F, et al. Frontoparietal Tracts Linked to Lateralized Hand Preference and Manual Specialization. Cereb Cortex. 2018;28(7):2482-2494.3. Hecht EE, Gutman DA, Bradley BA, Preuss TM, Stout D. Virtual dissection and comparative connectivity of the superior longitudinal fasciculus in chimpanzees and humans. Neuroimage. 2014;108:124-37. 4.Guglielmo Puglisi, Henrietta Howells, Tommaso Sciortino, Antonella Leonetti, Marco Rossi, Marco Conti Nibali, Lorenzo Gabriel Gay, Luca Fornia, Andrea Bellacicca, Luca Viganò ... Show moreAuthor Notes Frontal pathways in cognitive control: direct evidence from intraoperative stimulation and diffusion tractography Brain. 2019;142(8)2451–2465, 5. Jeffrey A. Fessler and Bradley P. Sutton. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Transactions on Signal Processing, 51(2):560–574, 2003.6. Tournier, J-Donald, Fernando Calamante, and Alan Connelly. "Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution." Neuroimage 35.4 (2007): 1459-1472. 6.Ben Jeurissen, Jacques-Donald Tournier, Thijs Dhollander, Alan Connelly, and Jan Sijbers. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion mri data. NeuroImage, 103:411–426, 2014.7. Garyfallidis, Eleftherios, et al. "Dipy, a library for the analysis of diffusion MRI data." Frontiers in neuroinformatics 8 (2014):8. Maffei C, Yendiki A. Using HCP data to improve diffusion tractography in routine-quality data: Application to the virtual dissection of the SLF system. Proc. Intl. Soc. Mag. Res. Med., pp. 734, 2019Figures
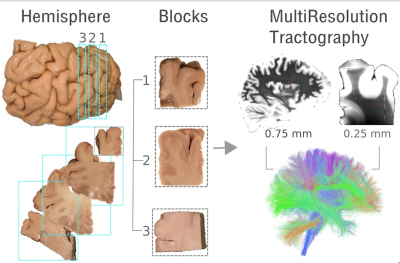
Figure 1. A fixed left hemisphere, that had been previously scanned (0.75 isotropic resolution), was cut into 1cm thick slabs and 3 smaller blocks were extracted along the superior frontal gyrus. These were scanned in a small bore at 0.25mm isotropic resolution. A MultiResolution tractography algorithm was implemented in order to incorporate the information provided by the higher-resolution data into the hemisphere.
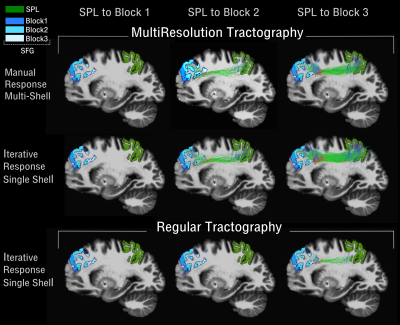
Figure 2. Tractography reconstruction of the SLFI using MultiResolution (top) vs Regular (bottom) Tractography. Tractography was performed using FODs estimated in two different ways for the high-resolution data: 1) iterative response function estimation on the highest shell and 2) manual response function on the multi-shell data. The SLFI was constrained to go through the superior parietal white matter (green) (SPL) and reach either block1(blue), block2(lightblue), or block3(white) along the superior frontal gyrus (SFG).