4465
A novel ex vivo structural connectome atlas of the C57Bl6 mouse brain using ultra-high field diffusion MRI at 17.2T1NeuroSpin, CEA, Gif-sur-Yvette, France, 2Centre Psychiatrie & Neurosciences, INSERM U894, Paris, France, 3Ecole Normale Supérieure Paris-Saclay & LAC-CNRS, Institut d’Alembert, Cachan, France, 4LBPA, Institut d’Alembert, Ecole Normale Supérieure Paris-Saclay, Université Paris-Saclay, Cachan, France, 5Département de Biologie, Ecole Normale Supérieure Paris-Saclay, Université Paris-Saclay, Cachan, France
Synopsis
Diffusion MRI is a powerful tool to investigate the structural connectivity of the brain. Ultra-high field preclinical MRI systems are equipped with strong gradients that allow to reach higher spatial and angular resolutions in animal models, enabling to segment white matter bundles in a similar way to what was achieved in humans. In this study, we propose to adapt the clustering approach of Guevara to rodents to establish a novel atlas of the connectivity of C57BI6 mice brains. 3D Hybrid diffusion imaging was performed ex-vivo allowing the reconstruction of a novel white matter atlas including 25 well-described fiber bundles.
Introduction
Diffusion MRI (dMRI) is a powerful tool to investigate the structural connectivity of the brain and to characterize its microstructure. Ultra-high field preclinical MRI systems are equipped with very strong gradients that allow to reach much higher spatial and angular resolutions in animal models, thus offering the possibility to segment white matter bundles in a similar way to what was achieved previously in humans. In this study, we propose to adapt the massive clustering approach of Guevara1 to rodents to establish the foundations of a novel atlas of the structural connectivity of C57BI6 mice brains. To this aim, 3D Hybrid diffusion imaging2 (HYDI) was performed ex-vivo on C57Bl6 mouse brains allowing the automatic reconstruction of a novel white matter atlas including 25 well-described fiber bundles.Materials and methods
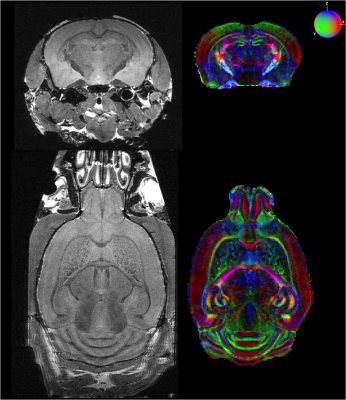
Acquisition Protocol – Experiments were performed on a 17.2T horizontal animal MRI system (Bruker BioSpin, Ettlingen, Germany) equipped with strong gradients (1000mT/m-9600T/m/s) with a dedicated mouse brain quadrature volume coil (Rapid Biomedical GmbH, Rimpar, Germany). A 3D diffusion-weighted Pulsed Gradient Spin Echo echoplanar (EPI) sequence was implemented with the following parameters: 13-shots, TR/TE=250/24.5ms, diffusion gradients characteristics δ/∆ = 5.0/12.3ms, three diffusion shells including 25/60/90 diffusion directions with b-values of 1500/4500/8000s/mm² and 17 b=0s/mm² reference images, isotropic resolution of 100µm, matrix size=192x152x152, total acquisition time of 26h. A 4h30min long 3D T2-weighted TurboRARE acquisition with a 60µm isotropic resolution was also performed for anatomical landmarks and registration purposes.Animal cohort – The MRI protocol was applied to eight ex-vivo C57Bl6 mice (4/4 females/males, 3 months old). After intracardiac perfusion (4% paraformaldehyde + Gd-DOTA), brains were collected, fixed by immersion and scanned on the 17.2T MRI system.
Pre-processing – Data were corrected from imaging artifacts using the Connectomist toolbox3 including denoising with a non-local means algorithm4 and correction of eddy currents.
Post-processing – For each individual, an affine 3D transformation was computed to match the DW dataset to the anatomical T2-weighted dataset. Orientation distribution function (ODF) maps were then computed using the analytical Q-ball model5 (spherical harmonics order 6, regularization factor 0.006). A deterministic regularized streamline tractography6 was performed to infer a dense whole brain connectogram per animal (~2.900.000 connections) using the following parameters: uniform seeding over a predefined domain of propagation computed from the average b=0s/mm² image (8 seeds per voxel), forward step: 20µm, aperture angle: 30°.
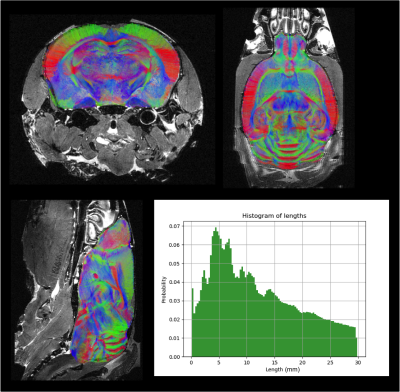
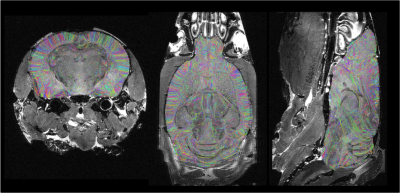
Intra-subject fiber clustering – After sub-dividing the individual tractograms into 4 subsets (inter-hemispheric, right/left hemispheres, cerebellum fibers), and 10 length groups (0-3/3-6/6-9/9-12/12-15/15-18/18-21/21-24/24-27/27-30mm), a density mask was computed for each group with a 100µm resolution, allowing to perform a hierarchical clustering of the connectivity matrix established from a random parcellation obtained within each density mask using a K-Means algorithm (minimum cluster size of 300 voxels, average cluster size of 2000 voxels, connectivity matrix threshold of 1%). All fibers intersecting a given cluster by more than 33% of their length were assigned to a target fascicle, yielding an individual set of fascicles for each mouse. For each fascicle, a centroid was defined corresponding to the fiber being closest to all the other fibers populating the fascicle, thus providing a map of centroids for each individual.
Inter-subject fiber clustering – A diffeormorphic transformation based on the Symmetric Normalization (SyN)7 approach provided by ANTs8 was computed to match each individual DW dataset and fascicle map to the frame of the Barrière9 C57Bl6 mouse brain atlas (including 1318 regions). A second hierarchical clustering step was then performed at the level of the population to automatically regroup centroids stemming from different mice into clusters and to only keep the most representative ones (present in at least 50% of the population).
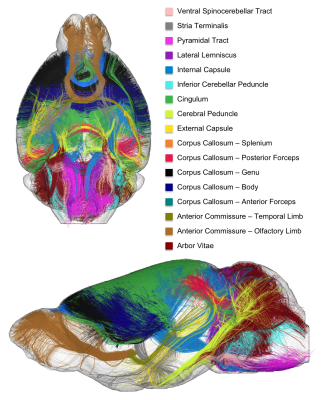
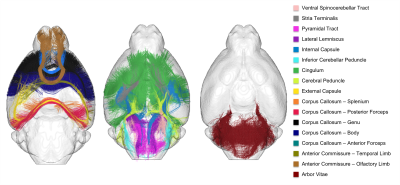
Atlasing – The final step consisted in selecting clusters intersecting the WM regions provided in the Barrière atlas to establish the novel C57Bl6 mouse brain white matter atlas: arbor vitae, olfactory and temporal limbs of the anterior commissure, anterior and posterior forceps of the corpus callosum, its body, genu and splenium, external capsule, cerebral peduncle, cingulum, inferior cerebellar peduncle, internal capsule, lateral lemniscus, pyramidal tract, stria terminalis and ventral spinocerebellar tract (the last 8 regions being present in both hemispheres).
Results & Discussion
Fig.1 provides a rendering of the anatomical T2-weighted and DW dataset acquired on the 17.2T scanner, assessing the high SNR level (>6) at 100µm even at b=8000s/mm2. Fig.2 gives a 3D rendering of an individual connectogram assessing the presence of fine white matter fascicles obtained thanks to the ultra-high resolution and depicts the histogram of fiber lengths. Fig.3 represents the map of the overall centroids obtained for one individual. Fig.4 provides a rendering of the novel C57Bl6 mouse brain white matter atlas including 8 lateralized bundles, 8 inter-hemispheric bundles and 1 cerebellum bundle. Fig.5 represents the inter-hemispheric/cerebellum/right/left hemisphere bundles allowing to visualize their differences in shape.Conclusion
In this study, we showed the successful measurement of ex-vivo ultra-high-resolution whole-brain 3D diffusion in eight C57Bl6 mice and we established the foundation of a novel connectivity atlas of the C57Bl6 mouse brain including 25 well characterized white matter bundles. Future work will consist in increasing the size of the cohort to add further finer white matter bundles and to offer a complete set of well-characterized connections in mice.Acknowledgements
No acknowledgement found.References
1. Guevara, P. et al., 2011. Robust clustering of massive tractography datasets. NeuroImage.
2. Wu, Y. C., & Alexander, A. L. (2007). Hybrid diffusion imaging. NeuroImage. https://doi.org/10.1016/j.neuroimage.2007.02.050
3. Duclap, D. et al., 2012. Connectomist-2.0: a novel diffusion analysis toolbox for BrainVISA. In 29th ESMRMB. Lisbone, Portugal
4. Buades, A., Coll, B., & Morel, J.-M. (2011). Non-Local Means Denoising. Image Processing On Line, 1, 208-212. https://doi.org/10.5201/ipol.2011.bcm_nlm
5. Descoteaux, M. et al., 2007. Regularized, fast, and robust analytical Q-ball imaging. Magnetic Resonance in Medicine
6. Perrin, M. et al., 2005. Fiber tracking in q-ball fields using regularized particle trajectories. Proceedings of the Information Processing in Medical Imaging conference
7. Avants, B.B. et al., 2008. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis
8. Avants, B.B. et al., 2012. A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. NeuroImage
9.
Barrière,
D.
A. et al., 2019. The
central orchestration of pregnancy and maternal care: a longitudinal
MRI study of morphometric changes that predicts the quality of
maternal behavior in mice. bioRxiv, under submission
Figures