4464
Diffusion Tensor Tractography of Tendons as a Tool for Assessing MRgFUS Ablation1Center for Image Guided Innovation and Therapeutic Intervention, Hospital for Sick Children, Toronto, ON, Canada, 2Radiation Oncology, University Health Network, Toronto, ON, Canada, 3Krembil Research Institute, University Health Network, Toronto, ON, Canada, 4Department of Surgery, Center for Image Guided Innovation and Therapeutic Intervention, Hospital for Sick Children, Toronto, ON, Canada
Synopsis
MRgFUS ablation of tendons is a promising treatment to disrupt tendons in conditions such as musculotendinous contractures. In this study, DTI and tractography were proposed as tools to assess tendons before and after MRgFUS ablation, providing a quantitative, directional, and visual assessment of tendon tract architecture and integrity. Diffusivity parameters (FA and ADC) changed in accordance to the tendon fascicle integrity. Fiber tractography visually delineated healthy tendon fascicles and confirmed tract disruptions at the same location as registered with b0 and T1-weighted images. This study demonstrates the potential for DTI and tractography as assessment tools for MRgFUS ablation treatments.
Introduction
Non-invasive ablation of tendons using Magnetic Resonance-guided Focused Ultrasound (MRgFUS) is a viable application that could precisely disrupt a tendon without the associated morbidities of surgery such as cutting through skin, or harming muscles, nerves, and arteries1,2,3. Ablation loosens musculotendinous contracture restoring some mobility and independence. Promising tools for monitoring MRgFUS ablation treatment in tendons are Diffusion Tensor Magnetic Resonance Imaging (DTI) and fiber tractography. They provide a quantitative, directional, and visual assessment of tendon tract integrity, superior to changes visualised in T1-weighted imaging4,5,6. Given that tendon fascicles are organized into tracts, diffusivity parameters, including fractional anisotropy (FA) and apparent diffusion coefficient (ADC), quantify changes following MRgFUS ablation7,8,9. Similarly, the architecture change and disrupted fascicle directionality can be mapped with tractography4,10,11. This study aims to apply and evaluate diffusivity parameters and fiber tractography as tools to assess tendons before and after MRgFUS ablation treatments.Method
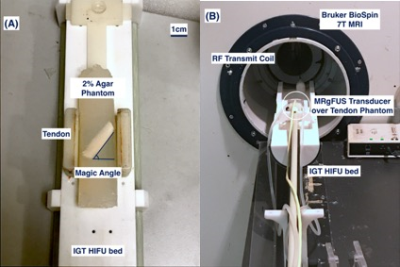
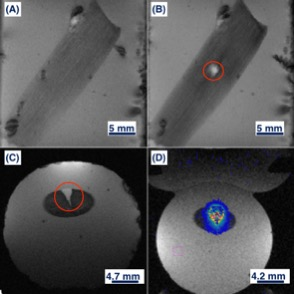
Ex-vivo porcine digital flexor tendons (n=4) were dissected, degassed, and embedded in 2% agar at the magic angle of 55o relative to Bo to maximize tendon T212,13. Tendons in agar were stored at 4oC, used within 24 hours, and equilibrated to room temperature prior to experimentation. Pre- and post-ablation imaging were acquired in a Bruker BioSpin 7T MRI with a 7.2 cm inner diameter RF transmit coil and anatomically shaped rat brain receiver coil (Figure 1). MRgFUS ablation was administered using a custom high-field small animal HIFU system (Image Guided Therapy, Bordeaux, France), at a frequency of 2.5 MHz at 8 W for 30 seconds, reaching a central temperature greater than 60oC for more than 20 seconds based on real-time MR thermometry achieving ablative thermal dosages. T1-weighted FLASH images were acquired in coronal and transverse plane (TR=100 ms, TE=6 ms, flip angle=30o, NEX=1, slice thickness=2 mm). DTI was performed using a 3D spin-echo segmented EPI technique (4 segments, TR=1200 ms, TE=26 ms, δ/Δ =3.5/10 ms, NEX=1, 1 b0 image, 12 diffusion directions with Jones sampling scheme with b=400 s/mm2, 160x160x60 matrix over 32x32x24 mm FOV for 200x200x400 μm spatial resolution, 250 kHz effective bandwidth, 62 min acquisition).Diffusion tractography was constructed using DiffusionToolkit 0.6.4.1 and TrackVis 0.6.1 for Linux 4.8.0-53, without distortion corrections. Tracking used a tensorline propagation algorithm, and an angle threshold of 35°. The treatment volume segmentation was performed using MIPAV 9.0.0 by a senior surgical resident. These volumes were defined visually by the contrast visible after MRgFUS ablation in the T2-weighted b0 images, and verified for consistency across the T1-weighted, ADC, and FA maps. Statistical analysis compared the FA and ADC averages (+/-standard deviation) pre- and post- ablation in the tendons within (n=4) and distal (n=4) to the ablated volume (AV). Thus, this study compared 4 groups: pre-ablation within AV, post-ablation within AV, pre-ablation distal to AV, post-ablation distal to AV. A one-way ANOVA and student’s t-test determined any difference within and between groups. Statistical significance was assumed at p<0.05.
Results
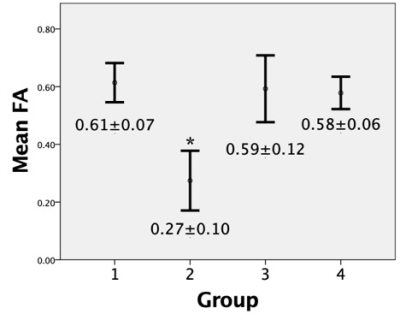
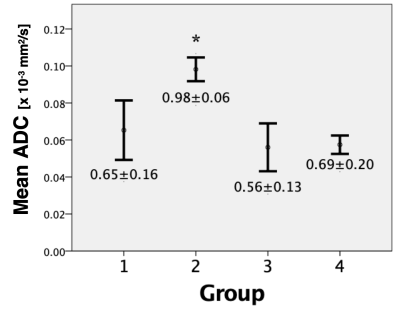
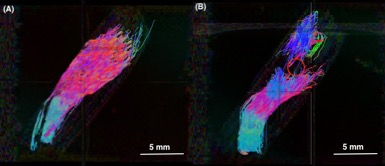
Within the ablation volume, FA and ADC values for pre-ablated, compared to post-ablated tendons, were found to be significantly different; FA pre-ablation=0.61±0.07 and post-ablation=0.27±0.10 (p<0.005), ADC pre-ablation=0.65±0.16x10−3 mm2/s, and post-ablation=0.98±0.06 (p<0.05). In tendons distal to the AV, there was no significant difference found in either FA or ADC when comparing pre- and- post ablation; FA pre-ablation=0.59±0.12 and post-ablation=0.58±0.06, ADC pre-ablation=0.56±0.13 and post-ablation=0.69±0.20 (both p>0.05). Also, there was no significant difference in FA and ADC within groups (p>0.05) (Figure 2 and Figure 3). Using fiber tractography, visual tendon tract disruption at the level of MRgFUS treatment was visually confirmed in all tendons (Figure 4). Similarly, T1-weighted images delineated the ablated volumes (Figure 5).Discussion
This is the first study that investigates changes in diffusivity parameters and tendon tractography following MRgFUS ablation. The decrease in FA and increase in ADC within the treatment volume following MRgFUS ablation were consistent with changes in anisotropy after disruption of tendon fascicles and loss of microstructural integrity5,7,8,9. No statistical differences were observed in either FA or ADC in tendons pre-ablation within the AV, and distal to the AV pre- and post-ablation, indicating there were no tendon structural changes in these regions (Figure 2 and Figure 3). Additionally, diffusion tractography in pre-ablation tendons demonstrated intact and continuous tendon fascicles. After ablation, these fascicles were disrupted, and the tracts were disorganized and discontinued at the level of ablation (Figure 4).Conclusion
This preliminary feasibility study showed that diffusivity parameters and tractography can be used as a quantitative, directional, and visual tools to evaluate tendons before and after MRgFUS tendon ablation. The decrease in FA and increase in ADC were quantitative measures of tendon fascicle integrity disruption. Tractography provided a visual representation for the loss of integrity and directionality in tendons.Future work include increasing the sample size, validating intra-observer and inter-observer reliability, analyzing axial diffusivity and radial diffusivity, and applying these tools for treatment dosage validation. Clinically, DTI and tractography could assess tendon integrity pre- and post-MRgFUS ablation in surgically treated conditions, such as musculotendinous contracture in spinal cord injury or cerebral palsy,14 idiopathic toe walking,15 and Achilles tendon lengthening for diabetic foot.16,17 DTI and tractography are promising evaluation tools for non-invasive treatments of conditions currently treated surgically.
Acknowledgements
Funding for this project has been possible in part by:
- Canadian Institutes of Health Research (CIHR)
- Natural Sciences and Engineering Research Council of Canada (NSERC)
- Ontario Graduate Scholarship
- The Government of Ontario Graduate Scholarships in Science and Technology
- SickKids Hospital Research Training Competition Scholarship
- Peterborough K.M. HUNTER Charitable Foundation
References
1. Budzik J-F, Balbi V, Verclytte S, Pansini V, Thuc V Le, Cotten A. Diffusion Tensor Imaging in Musculoskeletal Disorders. RadioGraphics [Internet]. 2014;34(3):E56–72. Available from: http://pubs.rsna.org/doi/10.1148/rg.343125062
2. Maxwell A, Sapozhnikov O, Bailey M. Disintegration of tissue using high intensity focused ultrasound: two approaches that utilize shock waves. 2012;8(4):24–37. Available from: http://scitation.aip.org/content/asa/journal/atdy/8/4/10.1121/1.4788649
3. Yeh CL, Li PC, Shih WP, Huang PS, Kuo PL. Imaging monitored loosening of dense fibrous tissues using high-intensity pulsed ultrasound. Phys Med Biol. 2013;58(19):6779–96.
4. Van Dyck P, Froeling M, De Smet E, Pullens P, Torfs M, Verdonk P, et al. Diffusion tensor imaging of the anterior cruciate ligament graft. J Magn Reson Imaging. 2017;46(5):1423–32.
5. Hara Y, Ikoma K, Kido M, Sukenari T, Arai Y, Fujiwara H, et al. Diffusion tensor imaging assesses triceps surae dysfunction after Achilles tenotomy in rats. J Magn Reson Imaging. 2015;41(6):1541–8.
6. Bihan D Le, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion Tensor Imaging: Concepts and Applications. 2001;546:534–46.
7. Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27(1):1–7.
8. Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: Theoretic underpinnings. Am J Neuroradiol. 2008;29(4):632–41.
9. Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion Tensor MR Imaging and Fiber Tractography: Technical Considerations. Am J Neuroradiol. 2008;29(5):843–52. Available from: http://www.ajnr.org/cgi/doi/10.3174/ajnr.A1052
10. Khalil C, Budzik JF, Kermarrec E, Balbi V, Le Thuc V, Cotten A. Tractography of peripheral nerves and skeletal muscles. Eur J Radiol [Internet]. 2010;76(3):391–7. Available from: http://dx.doi.org/10.1016/j.ejrad.2010.03.012
11. Sarman H, Atmaca H, Cakir O, Muezzinoglu US, Anik Y, Memisoglu K, et al. Assessment of Postoperative Tendon Quality in Patients With Achilles Tendon Rupture Using Diffusion Tensor Imaging and Tendon Fiber Tracking. J Foot Ankle Surg [Internet]. 2015;54(5):782–6. Available from: http://dx.doi.org/10.1053/j.jfas.2014.12.025
12. Wang N, Mirando AJ, Cofer G, Qi Y, Hilton MJ, Johnson GA. Diffusion tractography of the rat knee at microscopic resolution. Magn Reson Med. 2019;81(6):3775–86.
13. Wengler K, Tank D, Fukuda T, Paci JM, Huang M, Schweitzer ME, et al. Diffusion tensor imaging of human Achilles tendon by stimulated echo readout-segmented EPI (ste-RS-EPI). Magn Reson Med. 2018;(March):1–11.
14. Sutherland DH, Davids JR. Common gait abnormalities of the knee in cerebral palsy. Clin Orthop Relat Res. 1993 Mar;(288):139–47.
15. Levine MS. Congenital short tendo calcaneus. Report of a family. Am J Dis Child. 1973 Jun;125(6):858–9.
16. Tagoe MT, Reeves ND, Bowling FL. Is there still a place for Achilles tendon lengthening? Diabetes Metab Res Rev. 2016;32:227–31. Available from: http://libweb.anglia.ac.uk/
17. Frykberg RG, Bowen J, Hall J, Tallis A, Tierney E, Freeman D. Prevalence of equinus in diabetic versus nondiabetic patients. J Am Podiatr Med Assoc. 2012;102(2):84–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22461264
Figures